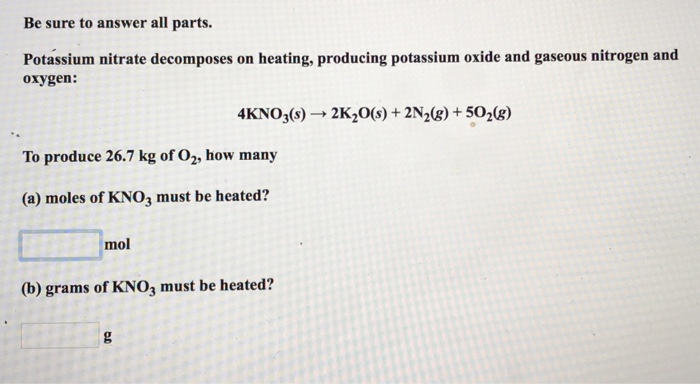Bringing the digestion tubeflask and mixture to a rolling boil about 370 o C to 400 o C using a heating a block. Potassium nitrite is an oxidizer and may ignite combustible materials wood paper oil etc.
What Happens When You Heat Potassium Nitrate Quora
Lift the thermometer and mark the KNO 3 solution level step 4 and 5.

Heating potassium nitrate. Potassium nitrate should be stored in a cool dry area away from combustible wood paper oil etc organic or other readily oxidizable materials. HOW TO DETERMINE IF YOU ARE BEING EXPOSED The. MICROWAVE HEATING INSTRUCTIONS Instructions developed using an 1100 watt oven.
The alchemists experimented with the sulfur and saltpeter heating the substances in order to transform them. If a positive result is obtained. Vulcan XC-72R carbon was supplied by Cabot Corp.
Medium-temperature collectors are also usually flat plates but are used for heating water or air for residential and commercial use. Magnesium nitrate is a crystalline source with high water solubility for use consistent with nitrates and lower acidic pH. 3 Basic salt normally aqueous bicarbonate of potassium potash sodium bicarbonate sodium carbonate sodium phosphate etc.
Of the three components charcoal is the easiest to obtain in the wild. Potassium nitrate may be shipped via air rail road and water in containers bearing the label Oxidizer. One way is to make your own ice cream in a baggie where adding salt to water produces a mixture so cold it can freeze your treat.
It was first isolated from potash the ashes of plants from which its name derives. Setup for heating the KNO 3 in test tube. 60 sodium nitrate and 40 potassium nitrate.
Even the nitrate products are oxidizing agents. THE THERMODYNAMICS OF POTASSIUM NITRATE DISSOLVING IN WATER VERSION V121113 OBJECTIVE. Nitrate compounds are usually soluble in water.
Be aware of the concentration units in the figures. 5 wt nafion solution in lower alcohols was purchased from Sigma-Aldrich. 4 Neutral salt normally aqueous calcium chloride calcium nitrate calcium sulphate magnesium chloride nitrate of potassium potassium sulphate sodium chloride sodium nitrate sodium sulphate etc.
Charcoal and its purpose in gunpowder. This is achieved by heating the KCl with metallic sodium to a temperature of 850 o. Potassium is a chemical element with the symbol K from Neo-Latin kalium and atomic number 19.
Silver nitrate AgNO 3 AR 998 Silver acetate AgC 2 H 3 O 2 AR 995 and potassium hexacyanocobaltateIII K 3 CoCN 6 98 were purchased from Aladdin. In 1776 Antoine-Laurent Lavoisier showed that it contained oxygen and in 1816 Joseph-Louis Gay-Lussac and Claude-Louis Berthollet established its chemical composition. The alchemist Wei Boyang wrote the Book of the Kinship of the Three detailing the experiments made by the alchemists.
It is used to make explosives matches fertilizer fireworks glass and rocket fuel. With fuels the reaction may be violent. Heating the mixture in the tubeflask until white fumes can be seen and then continuing the heating for about 60-90 mins.
Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. During the 8th century Tang dynasty. Molality moles of solutekg of water molliter.
Mark the solution level on the test tube with a sharpie and label it as 1. A common laboratory process used for many years ascribed to a German chemist Johann Rudolf Glauber 1648 consisted of heating potassium nitrate with concentrated sulfuric acid. Despite the lower electropositivity of sodium when compared to potassium.
Add carbon tetrachloride 1 mL and a few drops of freshly prepared chlorine water. Adding seven grams of potassium sulfate and a catalyst usually copper. Commercial PtC 20 wt was supplied.
For fires involving potassium nitrite extinguish with dry chemical CO2 Halon water spray. Wrap in paper towel. It can be noted that when the solid form of potassium.
CaCO 3 ----- CaO CO 2. Use Salt to Melt Ice Activity You can demonstrate the effect of freezing point depression yourself even if you dont have an icy sidewalk handy. Definitions are provided on page 5.
Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. By the way if its not actually the holidays and I havent updated this recently sorry about that. KCl is produced as a by-product during the synthesis of nitric acid from hydrochloric acid and potassium nitrate.
We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website. KCl Na NaCl K. For the purpose of gunpowder it provides a weak form of carbon more accurately carbon plus.
If you just want to see an example of how cold ice plus salt can get mix 33 ounces of ordinary table salt with 100. 30 Power 90 seconds. ALWAYS FOLLOW HEATING INSTRUCTIONS.
For small spills of potassium nitrate place material into a clean dry covered. Potassium hydroxide KOH 95 was obtained from Meryer. Calcium carbonate is decomposed from heat to form calcium oxide and carbon dioxide.
I promise that I wont take your. Mass of solutetotal mass of solution100 molkg. Saltpeter melts at 220 C 430 F and is kept liquid at 290 C 550 F in an insulated storage tank.
Also prolonged exposure to heat or fire may result in an explosion with the production of poisonous gases. Gunpowder consists of charcoal sulfur and potassium nitrate commonly known as saltpeter. Formation of a heavy white or yellow precipitate of silver halide indicates halogen.
Potassium Nitrate is a transparent white or colorless crystalline sand-like powder or solid with a sharp salty taste. Molarity moles of soluteliter of solution Values are tabulated below the figures. KEEP FROZEN UNTIL READY TO USE.
It is a type of decomposition in which a single reactant is broken down to more than one product with the help of heating. Charcoal and sulfur act as the fuels while saltpeter is the oxidizer more on oxidation below. St irrin g ro d T h e rmo me te r KN O 3 so lu tio n in te st tu b e Hot plate H o t w a te r b a th.
See also density of aqueous solutions of inorganic chlorides inorganic sodium salts some other inorganic substances organic acids and organic. Here are the rules for using this free website. Im glad you found your way here for chemistry help because youre in the right spot to be enlightened.
Heat times may need to be adjusted. The nitrate compounds can form a flammable mixture when combined with hydrocarbons. Thermal energy or heat energy is used to break the reactants.
This is our newest publication and has been created to support the school technician profession in Scotland. This is an endothermic reaction as heat is used to break the bonds of the reactants. Cool and add aqueous silver nitrate 1 mL compare with a blank.
The chemical equation for this reaction is. REASON FOR CITATION Potassium Nitrate is on the Hazardous Substance List because it is cited by DOT. Acidify the remaining portion of the fusion filtrate with dilute sulphuric acid boil and cool.
If additional time is needed microwave 5 seconds at a time until hot. Calcium nitrate can reduce the melting point to 131 C permitting more energy to be extracted before the.

When Potassium Nitrate Is Heated It Decomposes Into Potassium Nitrite And Oxygen Write A Balanced Youtube

Heating Of Potassium Nitrate Youtube

Solved Potassium Nitrate Decomposes On Heating Producing Chegg Com

Heating Potassium Nitrate Youtube
Solved Be Sure To Answer All Parts Potassium Nitrate Chegg Com

When Potassium Nitrate Is Heated It Decomposes Into Potassium Nitrite And Oxygen Write A Balanced Equation For This Reaction And Add The State Symbols Of The Reactants And Products
Solved Potassium Nitrate Decomposes On Heating Producing Chegg Com
Solved Potassium Nitrate Decomposes On Heating Producing Chegg Com

Potassium Nitrate Decomposes On Heating P Clutch Prep