Trace levels of EO and ethylene chlorohydrin ECH may remain on products after an EO sterilization process. Ethylene oxide was first developed in the early 19th century but wasnt commonly used as a chemical.

Ethylene Oxide Residual Sanichem Resources
The main side effect of using EO as a sterilization agent is that it can leave a residue on the devices being processed.

Ethylene oxide residuals. Gamma sterilization is used to sterilize human tissue grafts. LexaMed offers testing for AAMI Level 1 Minimum Fluid Barrier Protection Isolation Gowns and other barrier materials as defined in ANSIAAMI PB702012. Wastewater treatment sludge from the production of iron blue pigments T K008.
Biological evaluation of medical devices Part 7. Investigation into ethylene oxide treatment and residuals on DNA and downstream DNA analysis Sci Justice. It dissolves easily in waterEthylene oxide is a man-made chemical that is used primarily to make ethylene glycol a chemical used to make antifreeze and polyester.
Applicability of allowable limits for neonates and infants. Article Download PDF View Record in Scopus Google Scholar. The synthesized samples were characterized using X-ray powder diffractometry XRD field emission scanning electron microscopy FESEM energy-dispersive X-ray.
ISO 10993 - Replacing Sensitization Test with Systemic Toxicity Test. Overview of Ethylene Oxide EO or EtO Residuals. If the swab is sterilized using an ethylene oxide EtO method you should demonstrate that EtO and ethylene chlorohydrin ECH residuals meet the tolerable contact limits TCL specified in ISO 10993-7.
PCR and Quick Test swabs are sterilized with the carcinogen Ethylene Oxide. Ethylene oxide was first developed in the early 19th century but wasnt commonly used as a chemical. Investigation of double-layer and pseudocapacitance of surface-modi fi ed ionic liquid-functionalized graphene oxide.
The sterilization process is regulated so that medical devices sterilized using ethylene oxide are safe to use. Data on meeting these limits is established during the. This study initially demonstrated the effectiveness of ethylene oxide EO as a post-production treatment to eliminate DNA on swabs used as a sampling device for the recovery of cellular material.
Sterilization method Swab materials EtO for example ISO 11135 Gamma. Quietly injecting with. Wastewater treatment sludge from the production of chrome oxide green pigments anhydrous and hydrated T K007.
This method is preferred if sterilization is to be performed regularly. Other Medical Device Related. Other Medical Device Related Standards.
If the swab is sterilized using an ethylene oxide EtO method you should demonstrate that EtO and ethylene chlorohydrin ECH residuals meet the tolerable contact limits TCL specified in ISO 10993-7. Ethylene oxide sterilization residuals. Ethylene oxide sterilization residuals.
Because it is a strained ring ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Windebank AJ Blexrud MD. It is a cyclic ether and the simplest epoxide.
Biological evaluation of medical devices Part 7. Complete penetration can be achieved depending on the thickness of the material. EO Ethylene Oxide Residuals - EN ISO 10993-72008.
Insert device inappropriate autoclave pouch. EtO method you should demonstrate that EtO and ethylene chlorohydrin ECH residuals meet the tolerable contact limits TCL specified in ISO 10993-7. In this work a simple hydrothermal method was employed to prepare a pristine sample of copper oxide CuO and three samples of copper oxidegraphene nanocomposites CuO-xG with x 25 5 and 10 mg of graphene.
Gas sterilization by ethylene oxide may be used up to a maximum temperature of 65 C and 8psi. ISO10993-7 outlines the specific limits of EO and ECH that must not be exceeded in order to ensure product and patient safety. Ethylene oxide is a flammable gas with a somewhat sweet odor.
A three-membered ring consisting of one oxygen atom and two carbon atoms. ISO 10993-72008Amd 12019 Biological evaluation of medical devices Part 7. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor.
Do not exceed a temperature of 135 C and a pressure of. Subsequently the potential adverse effects of any residual. Consideration Ethylene Oxide Radiation Sterilization dose determination Overkill or bioburden methods Bioburden methods Temperature Typical 37-63 C Typical ambient to 20 C Pressure RH.
The gamma irradiation process does not create residuals or impart radioactivity in the processed items. Occupational Safety and Health Administration 2002. Contract Laboratory and Consulting.
Ethylene oxide sterilization residuals EO Residuals United States Pharmacopeia USP Chapter Bacterial Endotoxins Test European Pharmacopeia EP Chapter 2614 Bacterial Endotoxins Japanese Pharmacopeia JP Chapter 401 Bacterial Endotoxins Test ANSIAAMI ST72. Ethylene oxide burns from improperly sterilized mammary implants. Residual ethylene oxide in hollow fiber hemodialysis units is neurotoxic in vitro.
Therefore this version remains current. Ethylene Oxide EO the. Commonly used swab materials compatible sterilization methods and appropriate biological indicators are described below.
Investigation into ethylene oxide treatment and. 27 28 Ethylene oxide treatment is generally carried out between 30 and 60 C 86 and 140 F with relative humidity above 30 and a gas concentration between 200 and 800 mgl. Occupational Health and Safety Administration.
Sterilizing medical devices with ethylene oxide EO is a common practice primarily due to its extensive material compatibility. 2011 R 2016 Bacterial Endotoxins New draft document DIS11737-3 in progress Bacterial Endotoxin Test LAL. Ethylene oxide and.
Investigation into ethylene oxide treatment and. Ethylene oxide is an organic compound with the formula C 2 H 4 O. Ethylene oxide treatment is the most common chemical sterilization method used for approximately 70 of total sterilizations and for over 50 of all disposable medical devices.
Phys 153 2015 pp. Does anyone know of a biocompatible ABS tested certified to ISO 10993-1. A small amount less than 1 is used to control insects in some stored agricultural products and a very small amount is used in hospitals to sterilize medical.
Oven residue from the production of chrome oxide green pigments T Organic chemicals. Commonly used swab materials compatible sterilization methods and appropriate biological indicators are described below. 1 Wilson-Wilde et al.
The residues that may be found after processing are as follows. Connective tissue allograft such as bone cartilage tendons. F032 Wastewaters process residuals preservative drippage and spent formulations from wood preserving processes generated at plants that currently use or have previously used chlorophenolic formulations except potentially cross-contaminated wastes that have had the F032 waste code deleted in accordance with Section 26135 ie the newly promulgated equipment cleaning or replacement.
Steam sterilization can also be performed. Ethylene oxide sterilization residuals Amendment 1. 2017 Investigation into ethylene oxide treatment and residuals on DNA and downstream DNA analysis.
EU Medical Device Regulations. Commonly used swab materials compatible sterilization methods and appropriate biological indicators are described below. In summary only residual ethylene oxide remains on nasal swabs after sterilization.
Distillation bottoms from the production of acetaldehyde from ethylene T K010. Graphene oxide powders with different oxidation degree prepared by synthesis variations of the Hummers method. Buy this standard This standard was last reviewed and confirmed in 2016.
We then continued to discuss non-heat methods. ISO 10993-72008 specifies allowable limits for residual ethylene oxide EO and ethylene chlorohydrin ECH in individual EO-sterilized medical devices procedures for the.

Pdf Residual Ethylene Oxide In Medical Devices Effects And Estimation Methods An Overview
Ethylene Oxide Residuals Sterility Assurance Labs Sterility Assurance Labs

Ethylene Oxide Residue Testing In Food At Campden Bri

Pdf Residual Ethylene Oxide In Medical Devices And Device Material
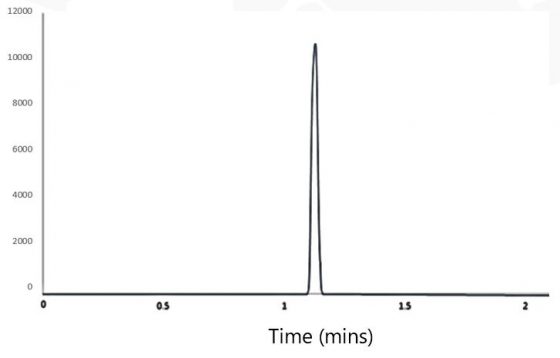
Detection Of Ethylene Oxide Eo Residue In Medical Protective Products

Overview Of Ethylene Oxide Residuals Techtip Steris Ast

Ethylene Oxide Residual Analysis
Ethylene Oxide Residuals Testing Lab In Delhi Baddi Bangalore