Sorbic Acid Contact
Custom Blending and Packaging. The Crossword Solver found 20 answers to the kind of acid crossword clue.
However cross contact is possible due to common handling and preparation areas in our restaurants.
Sorbic acid contact. Sorbic Acid and Salts. Enter the length or pattern for better results. Packaging To Meet Your Needs REQUEST MORE INFORMATION.
2016 Development of a predictive model for Salmonella spp. Talc CaprylicCapric Triglyceride PTFE Zinc Stearate Sodium Dehydroacetate Phenoxyethanol Sorbic Acid Tin Oxide EthyleneAcrylic Acid Copolymer Stearic Acid. Juneja V et al.
Contact dermatitis is a type of inflammation of the skin. Sodium-2-pyridinethiol-1-oxide sodium-omadine sorbic acid sorbitan monooleate span 80 sorbitan sesquioleate stearyl alcohol streptomycin sulphate strontium chloride sulfanilamide sulphur precipitated tantal tartrazine tea tree oil oxidized teledermatology. The View From Jamestown.
It is a colourless solid that is slightly soluble in water and sublimes readily. The rash is not contagious or life-threatening but it can be very uncomfortable. Acid Water Treatment Intermediates FertilizerCrop Nutrition Polymer Additives See All Products.
Sorbic or 24-hexanedioc acid is a compound with antifungal but not bactericidal properties. Enriched Bleached Wheat Flour Wheat Flour Niacin Reduced Iron Thiamine Mononitrate Thiamine Riboflavin Folic Acid Water Vegetable Shortening Interesterified Soybean Oil Hydrogenated Soybean Oil AndOr Palm Oil Contains 2 Or Less Of. Put your understanding of this concept to test by answering a few MCQs.
Nutrinova Sorbic Acid NXT Panosorb Sunett NXT Sweetener. The Crossword Solver finds answers to American-style crosswords British-style crosswords general knowledge crosswords and cryptic crossword puzzles. Reduction in meat jerky product with temperature potassium sorbate pH and water activity as controlling factors.
Las Colinas Blvd Suite 900N Irving Texas 75039 United States Phone 1-972-443-4000. 25 mg Hydrocortisone USP in a cream base consisting of purified water cetyl alcohol glycerin stearyl alcohol propylene glycol sodium lauryl sulfate cetyl palmitate and sorbic acid. The soluble sorbates are preferred when it is desired to use the preservative in liquid form or when aqueous systems are to be preserved.
Choose your region above Celanese - The chemistry inside innovation Industries. Iowa State University Midwest Grape and Wine Industry Institute. Contact Our Head Offices.
It is the property of a biofuel which indicates the quality of lubricity or rate of degradation when stored over some time with inference to stability and shelf life. Fromm Four-Star offers cats a variety of entrées to choose from each carefully prepared in controlled batches at our family facility using meat fish and an assortment of fruits and vegetables. Benzoic acid is frequently used at levels of 005 to 01 percent in conjunction with sorbic acid.
Sorbic acid is a hexadienoic acid with double bonds at C-2 and C-4. To know more such benzoic acid uses download the BYJUS the learning app. Lactic acid bacteria such as Leuconostoc oenos and.
Potassium sorbate prolongs the shelf life of foods by stopping the growth of mold yeast and. Test your knowledge on Uses Of Benzoic Acid. Contact urticaria should be distinguished from contact dermatitis where a dermatitis reaction develops hours to days after contact with the offending agent.
It is effective up to pH 65 but effectiveness increases as the pH decreases. A monthly podcast featuring The Chemical Companys View from Jamestown content from the eyes of TCC President Robb Roach GM of Sales AJ Petrarca LatAm Operations Manager Javier Ferndandez Marketing Manager Ben Sawicki and more. Sorbic acid is sparingly soluble in water sodium sorbate has better solubility and potassium sorbate is very freely soluble and can be used to produce 50 stock solutions.
Tortilla Grilled Marinated Chicken Secret Sauce Lettuce Cheddar Cheese. It is a hexadienoic acid a polyunsaturated fatty acid a medium-chain fatty acid and an alphabeta-unsaturated monocarboxylic acid. It has the chemical formula CH 3 CH 4 CO 2 H.
CI 77019 Mica CI 77891 Titanium Dioxide CI 77491 Iron Oxides CI 77000 Aluminum Powder CI 75470 Carmine CI 19140 Yellow 5 Lake CI 77510 Ferric Ferrocyanide. Water Treatment Products. The traditional route to sorbic acid involves condensation.
Hawkins is a business-to-business supplier manufacturer. Potassium sorbate has about 74 of the antimicrobial activity of the sorbic acid thus requiring higher concentrations to obtain the same results that pure sorbic acid provides. While rare allergic contact dermatitis may occur but ironically over-the-counter corticosteroid creams that contain sorbic acid are often the.
Sorbic acid or 24-hexadienoic acid is a natural organic compound used as a food preservative. The standardization of acid value of oil according to EN 141042003. Contact dermatitis results from either exposure to allergens allergic contact dermatitis or irritants irritant contact dermatitis.
It is an odorless and tasteless salt synthetically produced from sorbic acid and potassium hydroxide. It is commonly used in the food industry at doses of up to 200 mgL. The Contact Dermatitis Institute provides physicians and patients contact dermatitis research and information.
G2033552 C18-36 Acid Triglyceride Bis-Diglyceryl Polyacyladipate-2 Pentaerythrityl Tetraisostearate Polybutene Tridecyl Trimellitate Diisostearyl Malate Silica Dimethyl Silylate Phenoxyethanol Ethylhexyl Palmitate Pentaerythrityl Tetra-Di-T-Butyl Hydroxyhydrocinnamate Ethylhexylglycerin Calcium Sodium Borosilicate Sorbic Acid Calcium Aluminum Borosilicate Tocopheryl Acetate. Use cap to puncture seal. Potassium sorbate will produce sorbic acid once it is dissolved in water and is the most widely used food preservative in the world.
It has four geometrical isomers of which the transtrans-form is naturally occurring. It was first isolated from the unripe berries of the Sorbus aucuparia rowan tree hence its name. Contact urticaria is an immediate but transient localised swelling and redness that occurs on the skin after direct contact with an offending substance.
We are unable to guarantee that any menu item can be completely free of allergens. This was a brief on benzoic acid application. Celanese Europe BV The New Atrium 6th floor Strawinskylaan 3105 1077 ZX Amsterdam The Netherlands Phone 31 20721 3700 Celanese Sulzbach Commercial Center Am.
Tortilla Soft Tacos Kids Quesadillas. CaprylicCapric Triglyceride Talc. Salt Leavening Sodium.
2 to 4 applications daily. See package insert for full prescribing information. Celanese Corporation Headquarters 222 W.
Click Start Quiz to begin. Customers with allergies and sensitivities should exercise judgment when ordering. Click the answer to find similar crossword clues.
Sodium sorbate in solid form is unstable and very rapidly undergoes oxidation on. It is a conjugate acid of a. Europe Middle East and Africa.
Enriched Bleached Wheat Flour Wheat Flour Niacin Reduced Iron Thiamine Mononitrate Thiamine Riboflavin Folic Acid Water Vegetable Shortening Interesterified Soybean Oil Hydrogenated Soybean Oil AndOr Palm Oil. WHEAT flour water sugar yeast rapeseed oil WHEAT dextrose. Adhesives Additives Building Materials Carpet Formaldehyde-based Intermediates Hot Melt Adhesives PVB and PVC-based Resins Solvent Vinyl Foundations Water-based Adhesives Aerospace Commercial Aircraft and General Aviation Satellites Electronics and.
Each gram contains. The acid value is a measure of potassium hydroxide mg that is required to neutralize the free fatty acid contained in unit mass g of chemical substance. Some symptoms of contact dermatitis can include itchy or dry skin a red rash bumps blisters and swelling.
Tortilla Soft Tacos Kids Quesadillas Crispy Chicken Creamy Ranch Lettuce Cheddar Cheese. Degradation of sorbic acid does not cause substantial changes in the acid composition of wine but it does lead to the appearance of compounds with an aroma of geraniums. We provide known instances of allergens.
Sodium Hydroxide Flammable
Product Overview Caustic soda is an essential ingredient in many industrial and commercial applications. Sodium hydroxide is used to help manufacture a variety of medicines and pharmaceutical products from common pain relievers like aspirin to anticoagulants that can help to prevent blood clots to cholesterol-reducing medications.
Carbon steel is recognized as generally safe for the handling and storing of NaOH but will chemically attack this mild steel at elevated temperatures.

Sodium hydroxide flammable. Sodium Hydroxide 10 wv Product code. Metals such as aluminum tin. Eye contact can result in corneal damage or blindsides while skin contact can produce inflammation and blistering.
11 01292018 EN English US Page 1 SECTION 1. VZ4050000 LD50LC50 CAS 1310-73-2. Exposure can result in chemical burns as sodium hydroxide solutions can readily decompose body tissue.
WB4900000 CAS 497-19-8 sodium carbonate. Sodium hydroxide will chemically attack these metals releasing them into the solution where the high pH and presence of water leads to the generation of flammable and explosive hydrogen gas. Potassium hydroxide may be used as a substitute for sodium hydroxide but may cost more.
SODIUM HYDROXIDE Caustic Soda is a strong base. Individuals on a low sodium diet should consult a doctor before installing an injection system. Catalyzes the polymerization of acetaldehyde and other polymerizable compounds.
154 Environmental Hazard No information found. Reacts rapidly and exothermically with acids both organic and inorganic. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Store sodium hydroxide in a cool dry place away from flammable materials. Sodium hydroxide is also the most common base used in chemical laboratories. Sodium hydroxide reacts with metals aluminum lead tin or zinc to form flammable and explosive hydrogen gas.
BP359-212 CAS-No 1310-73-2 Synonyms Caustic soda Recommended Use Laboratory chemicals. 11 04032018 EN English US Page 1 SECTION 1. Sodium methylate is a white amorphous powder.
Caustic soda Lye Sodium hydroxide Soda lye Sodium hydrate Colorless to white odorless solid flakes beads granular form. Identification Product form. 102 103 Sodium spontaneously explodes in the presence of water due to the formation of hydrogen highly explosive and sodium hydroxide which dissolves in the water liberating more surface.
Its common name derives from its chemical identity as a sodium hydrate and because it is caustic or corrosive. Toxic fumes of sodium oxide. Fuel cells work like batteries to cleanly and efficiently produce.
It reacts with water to form sodium hydroxide a corrosive material and methyl alcohol a flammable liquidThe heat from this reaction may be sufficient to ignite surrounding combustible material or the sodium methylate itself if the water is present in only small amounts. Iii Background and acknowledgements The first United Nations Office on Drugs and Crime UNODC Guidelines for the Safe Disposal of Chemicals used in the illicit manufacture of Drugs STNAR36 was published in 2006 and was the culmination of an expert group meeting on. Marked set by housedj.
Store solutions of inorganic hydroxides in labeled polyethylene containers Flammable Solids Organic Bases PoisonsToxins Corrosive Bases-InorganicCaustics. It readily absorbs water and forms aqueous solutions. Sodium Hydroxide 50 ww CAS-No.
Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. An extremely violent polymerization results from contact of acrolein with alkaline materials such as sodium hydroxide Chem.
Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. Sodium hydroxide can form shock sensitive salts on contact with nitrogen containing compounds. Commercially available caustic soda or sodium hydroxide is usually sodium.
Corrosive to metals Exposure Routes inhalation ingestion skin andor eye contact Symptoms irritation eyes skin mucous membrane. Unmarked set by housedj. They are not combustible and do not support combustion.
Ingestion and contact with moisture on skin eyes or mucous membranes can cause severe burns. Sodium hydroxide reacts with strong acids hydrochloric sulfuric or nitric water and moisture to rapidly release heat. A violent polymerization results if acetaldehyde contacts alkaline materials such as sodium hydroxide.
Not combustibleUse extinguishing agent suitable for surrounding fire. Worldwide production in 1998 was around 45 million tonnes. Caustic soda is.
Caustic soda solutions are colorless and strongly alkaline. Sodium Hydroxide Flammable Liquids Violent Reaction Hydroxide with a spill tray away from DO NOT store under the sink. Sodium hydroxide also known as lye or caustic soda - Sodium hydroxide is very hazardous in case of skin or eye contact ingestion or respiration.
Readily absorbs moisture from the air to give caustic semi-solids that attack aluminum and zinc with the evolution of flammable hydrogen gas. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. These reactions can occur violently for example.
58 Monday March 26 2012 Rules and Regulations Date of issue. Sodium forms flammable hydrogen and caustic sodium hydroxide on contact with water. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH.
It is used to process edible fats and oils and to make other chemicals. Must be dated upon delivery and opening two dates Hydrofluoric. Sodium Hydroxide 10 wv Safety Data Sheet according to Federal Register Vol.
Sodium hydroxide solid Hazard Class. In the energy sector sodium hydroxide is used in fuel cell production. Relevant identified uses of the.
Specific Hazards Arising from the Chemical. It is a strong colorless alkali. PEARLSSODIUM HYDROXIDE MICRO PEARLS FOODSODIUM HYDROXIDE CHEM PURUMSODIUM HYDROXIDE EP PELLETSCAUSTIC SODA FLKCAUSTIC SODA MICROPEARLCAUSTIC SODA PEARL OGCAUSTIC SODA MICROPEARL SLY REACH registration number 01-2119457892-27 CAS number 1310-73-2 EU index number 011-002-00-6 EC number 215-185-5 12.
Safety Data Sheet SD-85 1961. May initiate polymerization in polymerizable organic materials. Product Name Sodium hydroxide Cat No.
Caustic soda is one of the common names for sodium hydroxide NaOH which is also known as lye. Sodium Hydroxide 50 ww Safety Data Sheet according to Federal Register Vol. Tin nitromethane leather flammable liquids organic halogens wool.
800-424-9300 CHEMTRECÒ Outside the USA. Sodium hydroxide caustic soda caustic lye Chemical Formula. Attacks aluminum and zinc with evolution of hydrogen a flammable gas.
Section 11 - Toxicological Information NIOSH RTECS CAS 1310-73-2 sodium hydroxide. Mixtures Product name. Exposure will leave the skin.
Contact with water causes violent frothing and spatteringReacts with metals to produce highly flammable hydrogen gas. This is our newest publication and has been created to support the school technician profession in Scotland. Uses advised against Food drug pesticide or biocidal product use Details of the supplier of the safety data sheet Emergency Telephone Number CHEMTRECÒ Inside the USA.
Mixtures Product name. Sodium Hydroxide in Energy. In pure form caustic soda is a waxy white solid.
Use manufacturer specifications to compare sodium levels in the treated water to levels consumed from other sources in the diet. Draize test rabbit eye. 58 Monday March 26 2012 Rules and Regulations Date of issue.
Is Potassium Flammable
Attacks aluminum and zinc to generate flammable hydrogen gas. Flammable solids self-reactive substances solid desensitised explosives and polymerizing substances.

Is Potassium Flammable Violent Reactivity Firefighter Insider
For fires involving potassium nitrate extinguish with dry chemical.

Is potassium flammable. Phosphorus white or yellow dry aluminum and magnesium alkyls. Substances which on contact with water emit flammable gases. 58 Monday March 26 2012 Rules and Regulations Date of issue.
Hazardous Combustion Products Potassium oxides Protective Equipment and Precautions for Firefighters As in any fire wear self-contained breathing apparatus pressure-demand MSHANIOSH approved or equivalent and full protective gear. Accidental release measures Personal Precautions Use personal protective. Along with sodium hydroxide NaOH KOH is a prototypical strong baseIt has many industrial and niche applications most of which exploit its caustic nature and its reactivity toward acidsAn estimated 700000 to 800000 tonnes were produced in 2005.
For small fires use dry chemical or carbon dioxide. Ratio by mass tested exhibits a mean burning time less than the mean burning time of a 32 mixture by mass of potassium bromate and cellulose. Marked set by housedj.
POTASSIUM HYPOCHLORITE SOLUTION is a powerful oxidizing agent. Never direct water jet straight at liquid ammonia. Move containers from the fire area if possible to do so without risk to personnel.
UN1987 and Isopropanol UN1219 are not subject to the requirements of this subchapter provided the following packaging marking and documentation. Glacial acetic acid 100 Acetic acid 80 Acetic anhydride Formic acid 85 Propanoic acid 100 also called Propionic acid. It looks and acts much like ice but it contains huge amounts of methane.
Pour the solution into an evaporating dish and place on a steam bath. For large fires use water spray fog or regular foam. POTASSIUM HYDROXIDE SOLUTION is a strong base dissolved in water.
It is a white salt which is soluble in water. Nitroparaffins Inorganic bases amines. Amines such as Ethanolamine Tributylamine etc.
Potassium Thiocyanate Safety Data Sheet according to Federal Register Vol. Substance Substance name. How this react in flooded labwhen potassium and sodium are present.
It is a hat shaped like a large yellow banana that encompasses the players entire head with only a hole for the face. M1252 SDS Revision Date. Substances liable to spontaneous combustion.
Formal Chemical Name IUPAC potassium O-pentyl carbonodithioate. Googulator August 7 2017 402 PM An interesting aside. And it exists in huge quantities in marine sediments in a layer several hundred meters thick directly below the sea floor and in association with permafrost in the Arctic.
Substances liable to spontaneous combustion. Oxygen liquid or enriched air Flammable gases liquids or solids such as acetone acetylene grease hydrogen oils phosphorous. Fire will produce irritating corrosive andor toxic gases.
It is deliquescent often appearing as a damp or wet solid. Reacts exothermically with all acids. Store solutions of inorganic hydroxides in labeled polyethylene containers Flammable Solids Organic Bases PoisonsToxins Corrosive Bases-InorganicCaustics.
M1252 - ANSI - EN POTASSIUM CARBONATE ANHYDROUS ALL GRADES _____ SDS No. Potassium carbonate is the inorganic compound with the formula K 2 CO 3. Some air fresheners contain p-dichlorobenzene which is a toxic irritant.
Potassium carbonate will dissolve in water forming liquid potassium carbonate which is an irritating and corrosive material. B Non-infectious specimens such as specimens of mammals birds amphibians reptiles fish insects and other invertebrates containing small quantities of Ethanol UN1170 Formaldehyde solution flammable UN1198 Alcohols nos. May form highly explosive NCl3 on contact with urea.
Potassium Hydroxide Oxidizers Gas Generation Sodium Hydroxide Flammable Liquids Violent Reaction Hydroxide with a spill tray away from DO NOT store under the sink. Charcoal briquettes when shipped in bulk Class 43 solids are likely to be spontaneously flammable or to release flammable or toxic gas at a rate greater than 1 liter per kilogram of the material per hour when they come into contact with water. It is known to occur on every continent.
Nitrites Potassium or sodium cyanide. 03-Jul-2017 _____ SECTION 3. Flammable gas means a gas having a flammable range with air at 20C 68F and a standard pressure of 1013 kPa.
Petroleum distillates are flammable irritate the eyes skin and lungs and may cause fatal pulmonary edema in sensitive individuals. Without enough vegetables. Metal hydroxides such as Sodium Potassium Calcium Nickel hydroxide Ammonium hydroxide Organic bases.
It contains potassium or potash theyre not identical but - scientists look away now - the terms are often used interchangeably and potassium is a vital nutrient for crops. The aerosol propellants used in some products may be flammable and may cause nervous system damage if inhaled. Just as it does in humans potassium regulates plants water balance so tissue is firm and juicy and has a part in transporting food within the plant and creating sugars and starches.
Liquid potassium carbonate is corrosive to aluminum _____ Print date. Evaporate to dryness to obtain a mass of red crystals. Heating or contact with acids produces highly toxic fumes of chlorine gas Sax 9th ed 1996 p.
Must be dated upon. 11 02142018 EN English US Page 1 SECTION 1. Sodium and potassium sulfides.
2 b Preparation of Potassium hexathiocyanatochromateIII K 3 CrNCS 6 Make an aqueous solution of potassium thiocyanate KSCN 25 g chrome alum KCrSO 4 212H 2 O 30 g using distilled water 10 cm 3. Also potassium nitrate presents an explosion risk when shocked or heated and may produce poisonous gases in a fire. We will be publishing Issue 1 of The School STEM Technician at noon on the 1st December 2021 via the SSERC website.
Non-flammable Lethal dose or concentration LD LC. Unmarked set by housedj. Potassium carbonate is the primary component of potash and.
Carbon disulphide is manufactured from hydrocarbons and sulfur and is a very flammable liquid which is therefore extremely hazardous to manufacture and transport. Contact with metals may evolve flammable hydrogen gas. This is our newest publication and has been created to support the school technician profession in Scotland.
Ignited a polyethylene container liner when mixed with potassium persulfate by release of heat and oxygen MCA Case History 1155. K K with our previously reported. While not flammable itself potassium nitrate is a powerful oxidizer and if it comes into contact with easily oxidizable substances it may react rapidly enough to cause ignition violent combustion or explosion.
Identification Product form. The Potassium Bonnet is a community-created cosmetic item for all classes. 03-Jul-2017 3 of 14 See Section 11.
Gas hydrates are a crystalline solid formed of water and gas. The electrochemical performances of the samples are examined in potassium metal cells within 2744 V vs. Examples of materials that are dangerous when wet.
Potassium carbonate is mainly used in the production of soap and glass. Oxalic acid Silver mercury and their salts. LD 50 median dose.
Potassium hydroxide is an inorganic compound with the formula K OH and is commonly called caustic potash. The Potassium Bonnet was contributed to the Steam Workshop. Damaged cylinders should be handled.
Mouseover cells to preview. Extract the solid via suction filtration using. Ammonia is a volatile compound that can irritate the respiratory.
Any chemical which in the 41 or 11 sample-to-cellulose ratio by mass tested exhibits a mean burning time equal to or less. Mason is the YouTuber Thunderf00t. Substances which in contact with water emit flammable gases.
Do not get water inside containers.
Lithium Burning From Contact With Water
Under standard conditions it is the lightest metal and the lightest solid element. In locations such as Pastos Chicas near the ArgentinaChile.
/2-12033975b6304be093692315b1ca340a.jpg)
Why Lithium Batteries Catch Fire
Tesla has bet big on them and built a.

Lithium burning from contact with water. In Salar de Atacama mining activities consumed up to 65 of the regions water causing havoc for local farmers. The fear of accident hinders the fully acceptance of. Water may not extinguish burning batteries but will cool the adjacent batteries and control the spread of fire.
Lithium hydride is an inorganic compound with the formula Li HThis alkali metal hydride is a colorless solid although commercial samples are grey. Whats more lithium can be extracted from ocean water where reserves are practically unlimited enough to fulfill the worlds energy needs for 6 million years. Stone is a chemical element with the symbol Li and atomic number 3.
Tim Crowley a vice president at Lithium Americas said the company would operate responsibly planning for example to use the steam from. Risk of fire and explosion on contact with combustible substances and water. But theres one stumbling block and its huge.
Lithium-ion batteries have taken over the world. Global inventory for tritium. Lithium from proven easily extractable land-based resources would provide a stock sufficient to operate fusion power plants for more than 1000 years.
The TR and TR-induced smoke fire and even explosion are the most common features during the accidents of lithium ion battery. The Lithium content is combined into other compounds which do not react with water. Elsewhere in South America Argentinians in the Salar de Hombre Muerto natural salt pan have expressed concerns over the lithium mining in the region citing contamination to streams and the irrigation of crops.
The burning reaction also tends to liberate the fluorine from the lithium salt typically LiPF 6 dissolved in the electrolyte. ADS CAS Article Google Scholar. Virtually all fires.
It is soluble and nonreactive with certain molten salts such as lithium fluoride lithium borohydride. Handbook of Reactive Chemical Hazards. According to the BLMs FEIS Lithium Americas plans to pump 17 million gallons of water from the aquifer each year which could leave less water to support the grasslands Bartells cattle graze.
It may be toxic by ingestion inhalation and skin absorption. Like all alkali metals lithium is highly reactive and flammable and is stored in mineral oil. It is used to make other chemicals.
Here are some lithium-ion battery safety tips to help businesses and their employees prevent workplace fires and injuries. Characteristic of a salt-like ionic hydride it has a high melting point and it is not soluble but reactive with all protic organic solvents. Wash skin with soap and water.
In the case of skin irritation or allergic reactions see a physician. Proponents of this technology note that hydrogen is more common than lithium by a long shot its literally the most abundant element in the universe and that it produces energy just as cleanly since the only end products of burning hydrogen are heat and pure water. Symptoms may be.
Lithium hydroxide solution appears as a clear to water-white liquid which may have a pungent odor. Sodium carbonate and sodium chloride are unsuitable to use as extinguishers for lithium fires since burning lithium will liberate the more reactive sodium in contact with them. After several months the water evaporates leaving a mixture of manganese potassium borax and lithium salts which is then filtered and placed into another evaporation pool.
E L Martín et al New constraints on the minimum mass for thermonuclear lithium burning in brown dwarfs Monthly Notices of the Royal Astronomical Society 2021. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structureThe chemical symbol for Lithium is Li. Many reactions may cause fire or explosion.
For every tonne of lithium produced 500000 gallons of water is used. Water immersion brought short circuit. If symptoms persist call a physician.
Of Power Sources 273 216222 2015. If symptoms persist call a physician. It is a soft silvery-white alkali metalUnder standard conditions it is the lightest metal and the lightest solid elementLike all alkali metals lithium is highly reactive and flammable and must be stored in vacuum inert atmosphere or inert liquid such as.
The amount of Lithium deposited during the Lithium plating when cells are damaged as described above is very small and not usually responsible for the fires which have occurred. The lithium extraction process uses a lot of waterapproximately 500000 gallons per metric ton of lithium. For most batteries the products typically consist of CO 2 and water vapor.
Lithium-Ion Battery Safety Tips for Employees. Butterworth-Heinemann Ltd 1990 p. Effects of exposure to Lithium.
The lithium plants which use vast amounts of water have exacerbated shortages. The most significant difference between lithium-ion and lithium-polymer batteries is the chemical electrolyte between their positive and negative electrodes. Furthermore many of the reported fires are due to burning electrolyte rather than the Lithium compounds.
Inhalation Move to fresh air. An experimental study on burning behaviors of 18650 lithium ion batteries using a cone calorimeter. Lithium production contributes to regional development and job creation in remote communities utilizes brackish non-potable water engages local communities with social programs generates material in-province tax revenue and facilitates the availability of electricity in remote and unconnected communities.
Contact may cause severe irritation to skin eyes and mucous membranes. An EV police car caught fire on the street. Always follow local state and federal regulations on proper battery disposal.
In case of fire where lithium batteries are present flood area with water or smother with a Class D fire extinguishant appropriate for lithium metal such as Lith-X. Eye Contact Rinse thoroughly with plenty of water also under the eyelids. The electrification of transportation is expected to cause a material reduction of.
Gives off irritating or toxic fumes or gases in a fire. To extract lithium miners drill a hole in salt flats and pump salty mineral-rich brine to the surface. Skin Contact Remove contaminated clothing and shoes.
Burning batteries will burn themselves out. Smoke fire and explosion are serious safety problems that arouse concerns from the public. Thacker Passs lithium is concentrated in a soft sedimentary clay which Lithium Americas intends to process with an experimental technique that involves burning hundreds of tons of sulfur.
Well burning hydrogen only produces water as a byproduct its exceptionally efficient and its much cleaner than lithium when it comes to producing it and recycling it at the end of the cars life. Workplace injuries from lithium-ion batteries are preventable with continual employee education. For the burning scenario the electrolyte burns efficiently producing primarily carbon dioxide CO 2 and water H 2 O as the by-products.
It is a soft silvery-white alkali metal. Right now we simply cannot manufacture enough. Most lithium-ion batteries unlike more traditional ones also include an electronic controller which regulates power and discharge flows so your battery doesnt overheat or explode.
Decomposition Of Bromoform In Light
Small spills of bromoform should be taken up. Kr for bromoform is 144Cmolal.
Bromoform should be stored in cool dark areas away from chemically active metals and strong caustics.
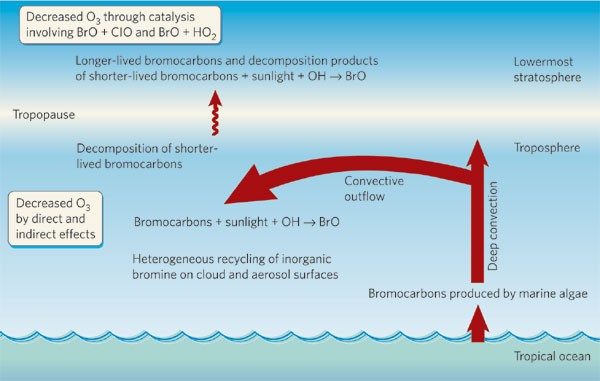
Decomposition of bromoform in light. P-listed codes and certain dioxin codes F020-F023 and F026-F028 are considered to be acute. Sep 21 2009 Cyclohexane C6H12 Bromine Br2 -- 1-Bromocyclohexane HBr. 287 289 For example Mediterranean grassland experiments where UV 289 291 or total solar radiation has been selectively.
Exposure to UV-A is associated with premature aging of the skin and some skin cancers. 2 M KCl and 10 drops. Bromine is the third halogen being a nonmetal in group 17 of the periodic table.
Methane is an odorless nontoxic flammable gas which has a boiling point of -1640C. The following is the List of Hazardous Substances prepared by the Director pursuant to Labor Code Section 6380. Condensed light ends spent filters and filter aids and spent desiccant wastes from the production of certain chlorinated aliphatic hydrocarbons by free radical catalyzed processes.
Chlorine dioxide gas can be absorbed by the skin where it damages tissue and blood cells. UV-A 315 to 400 nm visible light and other solar radiation are only weakly absorbed by the ozone layer. Bromoform See Benzene Bromoform See 12-Dichlorobenzene See Benzene See Bromoform Sources.
Acute exposure of the skin to chlorine that originates from the decomposition of chlorine dioxide causes irritations and burns. 950 n-Butyl. Gap-filled data are available for 53 sites.
It is not an official legal edition of the CFR. Natural gas is about 70-95 methane depending on the location in which it is obtained. 710 sec-Butyl acetate.
These chlorinated aliphatic hydrocarbons are those having carbon chain lengths ranging from one to and including five with varying amounts and positions of chlorine substitution T F026. Dec 07 2009 Ultrafast photolysis of bromoform CHBr3 with a 267 nm pulse of light followed by broadband transient electronic absorption identifies the photoproducts and follows their evolution in both neat bromoform and cyclohexane solutions. See 29 CFR 19101051.
STEL 1 ppm5 ppm Butanethiol. A short summary of this paper. In most instances the symptoms of primary irritation are observed shortly after exposure.
What is the empirical weight of the compound. The efficiency of ultraviolet UV light for primary disinfection of water depends on the intensity of the light and the length of time the. A laboratory preparation involves removing a bromine from bromoform using sodium arsenite.
INTRODUCTION 1-1 11 OBJECTIVE OF THIS MANUAL 1-1 12 BACKGROUND 1-2 13 REGULATORY CONTEXT 1-3 131 Disinfection Profiling and Benchmarking 1-7 14 USE OF DISINFECTANTS AS CHEMICAL OXIDANTS 1-8 15 How CHLORINE is ADDRESSED IN THIS GUIDANCE MANUAL 1-8 16 A SUMMARY OF ALTERNATIVE DISINFECTANT PROPERTIES 1-9 17 SELECTING A. Dec 07 2009 Ultrafast photolysis of bromoform CHBr3 with a 267 nm pulse of light followed by broadband transient electronic absorption identifies the photoproducts and follows their evolution in both neat bromoform and cyclohexane solutions. Only those codes applicable to the University of Maryland are listed Hazardous waste is any solid waste that either exhibits any of the characteristics of hazardous waste or is a listed EPA waste.
Cyclohexane has no pi-unsaturation and is therefore not nucleophilic. Note that this substance will attack some forms of plastics rubber and coatings. However some chemicals produce a delayed irritant effect because the chemicals are absorbed through the skin and then undergo decomposition within aqueous portions of the skin to produce primary irritants.
As pH increases the rate of decomposition of ozone is accelerated. The Code of Federal Regulations CFR is the official legal print publication containing the codification of the general and permanent rules published in the Federal Register by the departments and agencies of the Federal Government. Reckhow and Singer 2011.
It is produced by the bacterial decomposition of organisms in the absence of oxygen and is found in natural gas marsh gas bovine flatulence etc. The numeric value of 2445 in both formulae is the molar volume of air in litres at normal temperature and pressure NTP which is considered to be 25ºC and 1 atmosphere 101325 kPa or 760 mm Hg or 760 torr. 35 Full PDFs related to this paper.
Summary of Sampling Requirements for Physical and Chemical Parameters for Acceptability Aspects Parameter l. The Electronic Code of Federal Regulations eCFR is a continuously updated online version of the CFR. Academiaedu is a platform for academics to share research papers.
APHA 22nd ed2011 ADWG 34 Table D-3. Depletion of the ozone layer increases primarily the amount of UV-B radiation that reaches the surface Q16. 950 tert-Butyl acetate.
The substances on this list are subject to the provisions of Labor Code Sections 6360 through 63997 and Section 5194 in Title 8 of the California Code of Regulations. Full PDF Package Download Full PDF Package. A 1263 g sample of this compound was dissolved in 25 g of bromoform producing a solution that freezes at 44c the solvent has a freezing point of 78C.
Decomposition of plant litter via UV-induced processes has been shown to occur in arid-land ecosystems. Ethylene oxide epichlorohydrin hydroxylamines and the chemical mustard agents such as. It has been postulated that this decomposition process is responsible for a decrease in the classical formation of ozonation by-products eg aldehydes.
11 Consequently the results are best the bromine reagent is used in excess. Taste o Container Minimum Volume of Material Sample Glass-stoppered 500 mL Mode of Preservation Holding Time. 285 286 However new studies suggest that exposure to solar radiation can stimulate the breakdown of plant litter in a range of ecosystem and plant litter types.
Avoiding ozone depletion that would increase human exposure to UV-B radiation is a. What is the molality of the bromoform solution. Inhalation of chlorine dioxide gas causes coughing a sore throat severe headaches.
Eye exposure eyes to chlorine dioxide causes irritations watering eyes and a blurry sight. Molecular weights can be found in the NIOSH Pocket Guide to Chemical Hazards chemical supplier lists the NIST Chemistry WebBook or other online databases. Its properties are thus similar to those of fluorine chlorine and iodine and tend to be intermediate between those of the two neighbouring halogens chlorine and iodineBromine has the electron configuration Ar4s 2 3d 10 4p 5 with the seven electrons in the fourth and outermost shell acting as its valence.
2-Butanone Methyl ethyl ketone 78-93-3. 29 CFR 191019l 106-99-0. Kirk-Othmer Encyclopedia of Chemical Technology.
Mc Graw Hill-Water and Wastewater Engineering 2010 RETAi L EBook-Di Gi Book. Bromoform may be shipped via air rail road and water in containers bearing the label Keep Away From Food. Halomethane compounds are derivatives of methane CH 4 with one or more of the hydrogen atoms replaced with halogen atoms F Cl Br or IHalomethanes are both naturally occurring especially in marine environments and human-made most notably as.
See Butyl mercaptan. In addition EPA Hazardous Waste Codes are also classified as acute and non-acute.