Moles of H 2504 2. The total is 98.
What Is The Mechanism For The Acid Catalyzed Esterification Of Formic Acid And 2 Butanol Socratic
ROH Na OH RO Na HOH.

Is 2-butanol an acid or base. Most alcohols are slightly weaker acids than water so the left side is favored. D All of the above. 2-methylbutan-2-ol is a tertiary alcohol that is propan-1-ol in which both of the hydrogens at position 1 have been replaced by methyl groups.
That means the hydroxyl group is attached to the second carbon atom. As a result it transfers a proton to water. Any correct structure of.
02 moles x 32. Alkalosis is the opposite condition with blood pH being excessively high. Or the reaction in which the 2-butanol.
The elimination of water from an alcohol is called dehydration. Sodium salt of Jun 19 2020 The product formed when ferrous sulphate is heated is A Ferric oxide B Sulphur dioxide C Sulphur trioxide D All of the above Answer. The companys unparalleled raw material expertise is focused on the supply of essential oils and aromatic chemicals.
The Solubility Test Flowchart 2. Several assessments are included with the guidelines models databases state-based RSL Tables local contacts and framework documents used to perform these assessments. 0299 moles HCN.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. The index into Erowids collection of more than 20000 personal reports describing first-hand experiences with psychoactive plants and drugs. It has a role as a protic solvent.
Please use this book to increase your knowledge for the laboratory pratictioner. An acid dissociation constant K a is a quantitative measure of the strength of an acid in solutionIt is the equilibrium constant for a chemical reaction known as dissociation of acidbase reactions. 2-butanol is a clear organic secondary alcohol.
2-Butanol 2 4-DNP Test for Aldehydes and Ketones 1 acetates 1 Acetone 1 Acetone Test 1 acidimetry and alkalimetry 1 activation energy 1 Alternatives for Pesticides 2 Analysis of an antacid tablet 1 Andrews condition 1 antacid When the reaction involves in a titration does not satisfies the conditions for a direct titration to be performed 1 aryl 1 Baeyer Test 1. The product of this reaction is a stronger acid than water. Chemistry 1 decade ago.
Malolactic conversion also known as malolactic fermentation or MLF is a process in winemaking in which tart-tasting malic acid naturally present in grape must is converted to softer-tasting lactic acidMalolactic fermentation is most often performed as a secondary fermentation shortly after the end of the primary fermentation but can sometimes run concurrently with it. In the group shown below which of the following alcohols is are likely to yield a product where skeletal rearrangement has occurred when treated with sulfuric acid. 204 g CaCO3 4a.
In aqueous solution the equilibrium of acid dissociation can be written symbolically as. Finally base catalyzed hydrolysis of the phthalimide moiety and the esters followed by acidification and thermal decarboxylation produces an amino acid and phthalic acid not shown. The sodium salt of the acid as the acid is neutralized by the base.
2 2 Only functional group correct Slegs funksionele groep korrek Max. A Lewis acid-base reaction therefore rapidly occurs in which a pair of nonbonding electrons on a water molecule are donated to the carbocation to form a covalent C O bond. 3 An elegant procedure known as the Strecker synthesis assembles an alpha-amino acid from ammonia the amine precursor cyanide the carboxyl precursor and an aldehyde.
Marking criteriaNasienriglyne Whole structure correctHele struktuur korrek. Pore size 02 μm pore size 05 μm cartridge nominal length 5 in. How the EPA conducts risk assessment to protect human health and the environment.
The pH of blood is usually slightly basic with a value of pH 7365. HA H 2 O A-H 3 O. The primary aim of the cards is to promote the safe use of chemicals in the workplace.
This base was expanded upon more than two decades ago to include fragrance production. The cards are data sheets intended to provide essential safety and health information on chemicals in a clear and concise way. After placing five bonds between the atoms and adding the two.
03 g NH3. B Calculate the mole fraction of ascorbic acid in this solution. Plaque can create a local.
The ICSC project is a common undertaking between the World Health Organization WHO and. Amines that are insoluble in pure water will be soluble in acid due to the formation of an ammonium chloride salt. Silver Nitrate Test for Alkyl Halides and Carboxylic Acids The reaction of an alkyl.
A solution containing 815 g of ascorbic acid dissolved in 230 g of water has a density of 122 gmL at 55 C. A Calculate the mass percentage of ascorbic acid in this solution. Its very The concentration of an acid or base in solution can be determined by titration with a strong base or strong acid respectively.
The most common disorder in acidbase homeostasis is acidosis which means an acid overload in the body generally defined by pH falling below 735. When an alcohol is treated with sodium hydroxide the following acid-base equilibrium occurs. This base was expanded upon more than two decades ago to include fragrance production.
Solubility in 6M HCl is a positive identification test for bases. Because the cyclohexene has lower boiling point than the cyclohexanol the cyclohexene can be distilled as it forms. Ascorbic acid vitamin C C6H8O6 is a water-soluble vitamin.
It is usually An Arrhenius acidbase reaction occurs when a dissolved aqueous acid and a dissolved aqueous base are mixed together. This is supported by our long-standing relationships with a worldwide fabric of producers ensuring the greatest prospects for uninterrupted supply in markets that are often volatile. Because the slowest step of this reaction only involves t-butyl bromide.
This value is often referred to as physiological pH in biology and medicine. The companys unparalleled raw material expertise is focused on the supply of essential oils and aromatic chemicals. The difference in the R-2-butanol and the S-2-butanol has to do with the.
963 1 3 428 107 0. 567 X 1022 Molecules Of Glucose C6H1206. Recalling that water is a much better leaving group than hydroxide ion it is sensible to use acid.
1 10 -6. In step three the base HSO4 -removes a proton from the carbon adjacent to the positively charged carbon forming an alkene and regenerating the acid catalyst H2SO4. The main target users are workers and those responsible for occupational safety and health.
This is supported by our long-standing relationships with a worldwide fabric of producers ensuring the greatest prospects for uninterrupted supply in markets that are often volatile. Urine was analyzed immediately 1 2 8 and 9 hr after drinking during 2 hr 375 mlkg of beverages containing orange juice 15 or 40 ethanol and 1 gl of 1-propanol 2-propanol 1-butanol 2-butanol isobutyl alcohol or a mixture of 1-propanol isobutyl alcohol. Where HA is an acid that dissociates into A known as the conjugate.
3-methyl-3-pentanol 33-dimethyl-2-butanol 22-dimethylcyclohexanol A 3-methyl-3-pentanol only B 33-dimethyl-2-butanol only C 22-dimethylcyclohexanol only. Moles atomic weightgram weight. Dehydration of 2-methyl-2-butanol produces primarily 2-methyl-2-butene a tri-substituted alkene.
125 cm Code 7 EPDM rubber seal.
Acidulants such as Citric Acid Fumaric Acid Malic Acid Tartaric Acid etc can be added to lower the pH level to make the mold inhibitors more effective Sorbates PH. Assisted grinding with an equimolar amt.

Pdf Solubility Of Citric Malonic And Malic Acids In Different Solvents From 303 2 To 333 2 K
H 2 C 4 H 4 O 5.

Malic acid solubility. Jia Centers for Disease Control and. All of these acids are by-products of the metabolism of pyruvic acid. Unstable as seen during the stability testing.
Tartaric acid levels decrease with increasing ethanol concentrations due to the decrease in the solubility of its salt potassium bitartrate also known as cream of tartar. If more acid needs to be removed it is better to use the double-salt deacidification with calcium carbonate. PSMs promote plant growth via generating phytohormones such as auxins gibberellins cytokinins or polyamides 1 25 30 40Organic acids such as carboxylic glycolic malonic succinic fumaric and alpha-ketoglutaric acid that hasten the maturity and thereby enhance the ratio of straw as well as the total yield have also been recognized among phosphate solubilizers 6 9 31.
It was observed that the itraconazole L-malic acid cocrystal exhibited a similar dissolution profile to that of the marketed formulation 68. The first three acidulants are available both in powder and granular form while the rest two are liquid. Now you may have a good knowledge of the food additive citric acid E 330.
DL-tartaric acid is produced from the enzyme. For example malic acid has been shown to leach out divalent metal ions but requires an additional reducing agent to accelerate the process for dissolving the. Malic acid is an organic compound with the molecular formula C 4 H 6 O 5It is a dicarboxylic acid that is made by all living organisms contributes to the sour taste of fruits and is used as a food additiveMalic acid has two stereoisomeric forms L- and D-enantiomers though only the L-isomer exists naturallyThe salts and esters of malic acid are known as malates.
When we applied these proteins to our ELISA diagnostic assay they all keep showing highly specific reactivity and very low background. ALA was isolated by Reed in 1951 1 as an acetate replacing factor and its first clinical use dates from 1959 in the treatment of acute poisoning by Amanita phalloides also known death cap from mushrooms 2. Malolactic conversion also known as malolactic fermentation or MLF is a process in winemaking in which tart-tasting malic acid naturally present in grape must is converted to softer-tasting lactic acidMalolactic fermentation is most often performed as a secondary fermentation shortly after the end of the primary fermentation but can sometimes run concurrently with it.
Table 42 Various Acids Found in Food and Beverages. In another study by Childs et al fluoxetine HCl succinic acid cocrystal was found to exhibit an approximately twofold increase in aqueous solubility after only 5 min. Cocrystal of PZQ with MA was chem.
We are very satisfied with the qualities of these proteins. Has a high solubility in water 592 gL 20 C. Müller-Thurgau 1891 and Koch 1900 later attributed the presence of lactic acid bacteria to a reduction in the acidity of wines and shortly afterwards in 1901 Seifert reported that these bacteria were capable of degrading malic acid.
It has a role as a food acidity regulator and a fundamental metabolite. The American Dairy Science Association ADSA is an international organization of educators scientists and industry representatives who are committed to advancing the dairy industry and keenly aware of the vital role the dairy sciences play in fulfilling the economic nutritive and health requirements of the worlds population. Malonic acid is used for the introduction of an acetic acid moiety under mild conditions by Knoevenagel condensation and subsequent decarboxylation.
Biomatik started the protein production process from codon optimization and gene synthesis the solubility of proteins we got were significantly improved. Thus this technique is. Of citric acid CA malic acid MA salicylic acid SA and tartaric acid TA gained in cocrystal formation which all showed pH-dependent soly.
The name malic acid comes from the apples botanical genus name malus while lactic acid HC 3 H 5 O 3 is found in wine and sour milk products such as yogurt and some cottage cheeses. However the most sol. In general solubilityavailability of heavy metals for plant uptake and suitability of a site for phytoextraction are additional factors that should be considered in addition to suitability of plants before using phytoextraction for soil remediation.
α-lipoic acid ALA also known as 12-dithiolane-3-pentanoic acid or thioctic acid is a compound commonly found in mitochondria necessary for different enzymatic functions. For example when NaClaq. Solubility g100 mL H2O Formic acid HCO2H 101 8 Infinite Acetic acid CH3CO2H 118 17 Infinite Propionic acid CH3CH2CO2H 141 -21 Infinite Butyric acid CH3 CH22CO2H 164 -5 Infinite Valeric acid CH 3CH23CO2H 186 -34 5 Caproic acid CH3CH24CO2H 205 -3 1 Caprylic acid CH 3CH26CO2H 239 17 Insoluble Capric acid CH3CH28CO2H 270 32 Insoluble Lauric acid.
It provides leadership in scientific and technical support to. It is important to note that the double-salt technique favors the removal of tartaric acid rather than malic acid unless the initial concentrations of malic acid are double the concentration of tartaric acid. Examples include the preparation of cinnamic acid used for the production of the anti-inflammatory cinmetacin and 345-trimethoxycinnamic acid the key intermediate of the vasodilators cinepazet and cinepazide.
It is a 2-hydroxydicarboxylic acid and a C4-dicarboxylic acid. By neat grinding with amorphous. Equimolar cyclodextrin complexes prepd.
Grapes also contain acids mostly tartaric acid and malic acid but levels decrease during vinification. Storage temperature is key to ensuring the. More recent studies particularly from the 1970s onwards confirmed the importance of malolactic fermentation in reducing acidity essential in red wines.
Formamide 1 formic acid 2 urea 3 diaminomaleonitrile 4 glycine 5 alanine 6 valine 7 leucine 8 proline 9 serine 10 asparagine 11 aspartic acid 12 glutamic acid 13 lysine 14 histidine. Organic chelates such as citric acid and malic acid can also be used to improve phytoextraction of heavy metals from polluted soils. Solubility rules are very useful in determining which ionic compounds are dissolved and which are not.
DL-malic acid acetic acid and phosphoric acid are derived from chemical synthesis. Malic acid is a 2-hydroxydicarboxylic acid that is succinic acid in which one of the hydrogens attached to a carbon is replaced by a hydroxy group. Sorbates are the more effective preservative against a wider spectrum of food spoilage microorganisms than benzoates or propionates When used at common pH levels of mildly acidic foods pH 55 60.
Polycarboxylic Antiscalant and Dispersant. Polyacrylic acid PAA Water 02 M NaNO 3 001 M NaH 2 PO 4 adjusted to pH 7 PL aquagel-OH 25 5991-5783EN Poly-alpha-olefi n PAO TCB 0015 BHT PLgel 160 5990-6971EN Polyamide HFIP 20 mM NaTFA HFIPgel 40 5990-7978EN Polyaniline NMP 01 LiBr PLgel 80 5991-5814EN Polyanion acrylic acid sodium salt Water 02 M NaNO 3 001 M NaH 2 PO 4 adjusted to pH 7 PL aquagel-OH.
Polyacrylic Acid Sodium Salt C14h18na4o8 Pubchem
To compare the safety and efficacy of polyacrylic acid 02 PAA gel and polyvinylalcohol 14 PVA in the treatment of patients with dry eyes.

Polyacrylic acid sodium salt. Coated a thin alginate hydrogel film onto the surface of a polyacrylic acid-grafted-polyvinylidene fluoride PAA-g-PVDF filtration membrane via layer-by-layer self-assembly of sodium alginate and Cu 2. Consequently the proposed hydrogel. Sodium alginate NaC 6 H 7 O 6 n is a linear polysaccharide derivative of alginic acid comprised of 14-β-d-mannuronic and α-l -guluronic acids naturally occurring from marine brown algae.
Where P S cm 2 s 1 is the salt permeability. Several reports using the Na-alginate binder demonstrated stiffness superior to those of other conventional binders in Li Na and K ion batteries. IRGAFOS P-EPQ 1324-Di-p-methylbenzylidene sorbitol Isopropylboronic acid Fluorescent Brightener 121 2-methylbut-3-yn-2-amine NNNN-Tetrakis2-Hydroxypropyl- Ethylenediamine dibutyl phthalate tris2-ethylhexyl benzene-124-tricarboxylate dioctyl decanedioate tert-Butylferrocene.
Polyacrylic acid is a weak anionic polyelectrolyte whose degree of ionisation is dependent on solution pH. Performance analysis of desiccant cooling system using polyacrylic acid sodium salt desiccant wheel Sarra Belguith Zina Meddeb Romdhane Ben Slama Pages. The great cotton mills in Lancashire gave the obvious location for the dyestuff industry around Manchester the largest city in Lancashire.
Designed to meet the challenge of. PAA polyacrylic acid M w 40000 60000 powder and PMA polymaleic acid 50 in water were purchased from Shanghai macklin Biochemical Co Ltd. In its non-ionised form at low pHs PAA may associate with various non-ionic polymers such as polyethylene oxide poly-N-vinyl pyrrolidone polyacrylamide and some cellulose ethers and form hydrogen-bonded interpolymer complexes.
2-Phosphonobutane -124-Tricarboxylic Acid Sodium salt PBTCNa 4 Penta sodium salt of Amino Trimethylene Phosphonic Acid ATMPNa 5 Hepta sodium salt of Diethylene Triamine Penta Methylene Phosphonic Acid DTPMPNa 7 Potassium Salt of HexaMethylene-DiamineTetra MethylenePhosphonic Acid HMDTMPAK 6. Sodium hexametaphosphate SHMP is a threshold agent derived from the dehydration of orthophosphoric acid or its sodium salt. In the present work we have successfully developed a.
Sodium Salt Of Polyacrylic. Sustained release drug delivery system 1. Herein we constructed a new type of highly stretchable anti-freezing self-healable and conductive hydrogel based on chitosanpolyacrylic acid.
SBSHIRSAND MPharm 1st year Deptof Pharmaceutical MPharmPhd Technology HKES COPGLB. It is used to inhibit the formation of calcium carbonate and metallic sulphate scale. In certain cases where the safety assessment indicates concerns on the use of the free acids only the salts should be authorised by indicating in the list the name as.
Each adhesive patch contains 700 mg of lidocaine 50 mg per gram adhesive in an aqueous base. At low concentrations it provides excellent flow properties and exhibits synergistic behavior with salt. Polyacrylic Acid PAA 50.
It delivers excellent suspending capability and stabilizes insoluble conditioning agents commonly used in 2-in-1 shampoos. Commercial glasses for glass-ionomer cements are typically based on. Depending on the concentration of calcium and sulphate and depending on the CF equation 23 the dosage is.
Manufacturer of Chemicals in India. Carbopol Silk 100 polymer is a crosslinked polyacrylic acid that is polymerized in a toxicologically-preferred cosolvent system. Dielectric Constant k is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current.
This super-absorbent polymer SAP has the ability to absorb 100 to 1000 times its mass in water. Each adhesive patch contains 700 mg of lidocaine 50 mg per gram adhesive in an aqueous base. Co Polymer Of Aaamps.
The Journal of Prosthetic Dentistry is the leading professional journal devoted exclusively to prosthetic and restorative dentistryThe Journal is the official publication for 24 leading US. Similarly the strong chlor-alkali industry chlorine sodium hydroxide sodium carbonate in the Northwest of England developed because of local coal and salt mines and the proximity of a major canal leading to a main port of England. It is vital that glasses for ionomer cements should be basic ie capable of reacting with an acid to form a salt.
The large amount of ions inside the network had five functions for the proposed hydrogel including excellent mechanical behaviors high conductivity self-recovery self-healing and anti-freezing capability. Dihydroxyaluminum aminoacetate disodium edetate gelatin glycerin kaolin methylparaben polyacrylic acid polyvinyl alcohol propylene glycol propylparaben sodium carboxymethylcellulose sodium polyacrylate Dsorbitol tartaric acid. Co Polymer Of Maaa.
C SF and C SP g cm 3 are the salt concentrations in the solution on the feed and permeate sides of. High quality and leading manufacturer supplier trader exporter importer dealer distributor of Industrial Chemicals Cleaning Chemicals Pharma Chemicals Apis Bulk Drugs Water Swimming pool treatment chemicals. Eighty-nine patients with dry eyes were randomly allocated to treatment with either PAA 48 or PVA 41 in a prospective investigator-masked study in two centers.
Dihydroxyaluminum aminoacetate disodium edetate gelatin glycerin kaolin methylparaben polyacrylic acid polyvinyl alcohol propylene glycol propylparaben sodium carboxymethylcellulose sodium polyacrylate D-sorbitol tartaric acid and urea. It also contains the following inactive ingredients. It is most widely used because it offers good inhibition at a low cost.
It also contains the following inactive ingredients. Sodium polyacrylate also known as waterlock is a sodium salt of polyacrylic acid with the chemical formula CH 2 CHCO 2 Na n and has broad applications in consumer products. Conventional drug administration often requires high dosages or repeated administration to stimulate a therapeutic effect which can lower overall efficacy and patient compliance and result in severe side effects and even toxicity 13For example intravenously administered Interleukin-12 IL-12 resulted in systematic toxicities including deaths in a clinical trial 4.
As the salts usually are transformed in the stomach to acid alcohol or phenol the use of salts with cations that have undergone a safety evaluation should in principle be authorised together with the acid alcohol or phenol. A SEMINAR ON SUSTAINED RELEASE DRUG DELIVERY SYSTEM presented by Under The Guidance Of MANE PRASHANT P. The monthly publication features timely original peer-reviewed articles on the newest techniques dental materials and research findings.
Lithium hydroxide sodium hydroxide. The parameters assessed were daily frequency of instillation of the study medications. In principle several different glass compositions can be prepared that fulfil this requirement but in practice only alumino-silicate glasses with fluoride and phosphate additions are fully satisfactory.
Sodium polyacrylate is an anionic polyelectrolyte with negatively charged carboxylic groups in the main chain.
Bipolar membrane and electrodialysis are used widely. Articles that describe this calculator.

Is Lioh An Acid Or Base Strong Or Weak Lithium Hydroxide
2 mgm3 NaOH NIOSH.
Is lioh an acid. Arrhenius theory is limit to the aqueous solution which means the presence of water solution is a must for knowing the acidic or basic nature of the substance. If the acid is 100 percent dissociated in solutions of 10 M or less it is called strong. A strong acid is one that dissolves in water.
Ternary acids commonly contain hydrogen a nonmetal and oxygen. It is a crystalline organic carboxylic acid with keratolytic bacteriostatic and fungicidal properties. Classify each substance as a strong acid strong base weak acid or weak baseDrag the appropriate items to their respective binsLiOH HF H2SO4 CH3COOH HClO4 NaOH CaOH2 NH3 HBr HCOOHCsOH HNO2 HI HCN KOH CH32NH BaOH2 HNO3 HCl CH3NH2 Strong Acid Weak Acids Strong Bases Weak BasesPart BWhat salt is produced in each of the following.
Find step-by-step Chemistry solutions and your answer to the following textbook question. Barium hydroxide These bases completely dissociate in solutions of 001 M or less. Share my calculation.
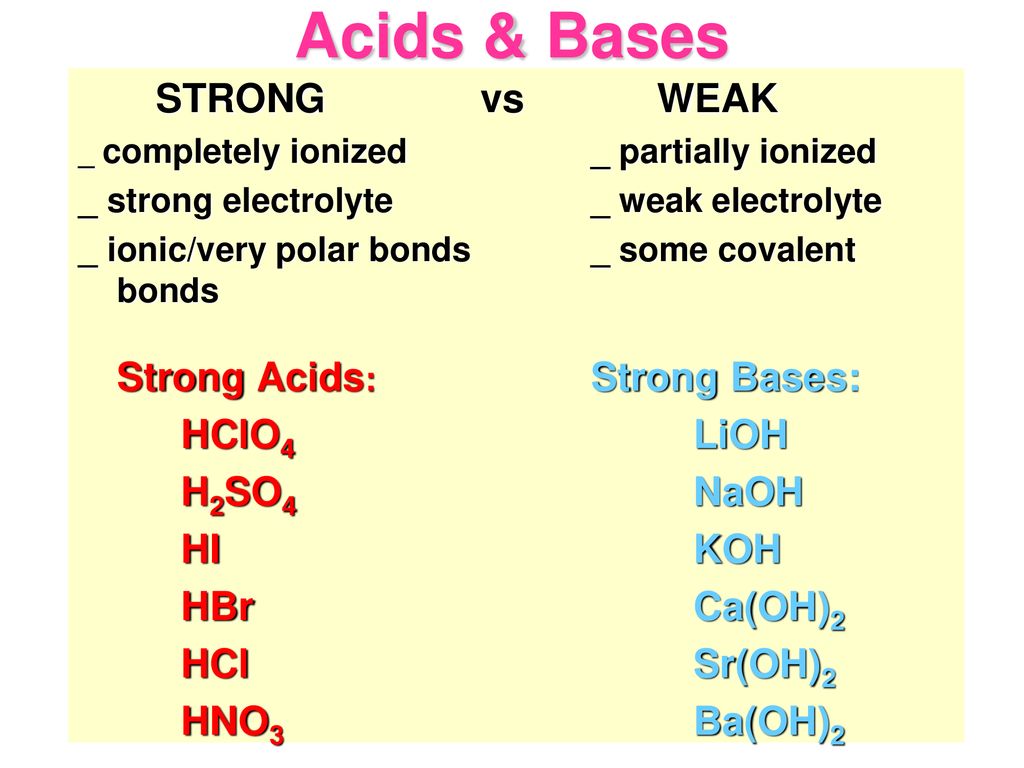
There are seven strong acids. Compiled from Appendix 5 Chem 1A B C Lab Manual and Zumdahl 6th Ed. Diethylamine CH 3 CH 2 2 NH.
15 February 1984 Issue 2. C 6 H 5 NH 2. Selenous acid 7 sulfuric acid - arsenic acid 8 phosphorous acid - nitrous acid 9 hydrofluoric acid - hydroselenic acid 10 hypoiodous acid - periodic acid 11 iodic acid - bromous acid Name the following bases.
I can complete and balance a neutralization equation and identify the salt. Is the Arrhenius base as they release OH ions when dissolved in an aqueous solution hence increase the concentration of OH ions. The common strong bases and their aqueous ions are.
H 2 CO 3. Group I Pesticide ACGIH. Strontium hydroxide BaOH 2.
The solute is assumed to be either strong acid or strong base. Classify each substance as a strong acid strong base weak acid or weak base. Formula Type Chemical Name CaO I Calcium oxide C 2 H 2 M Dicarbon dihydride LiOH I Lithium hydroxide SO 3 M Sulfur trioxide.
Create a System of Equations One Per Element H. Acid base production from sodium sulfate in single plant. Why CaOH 2 is a strong base.
For example Hydrochloric acid HCl Sulphuric Acid H 2 SO 4 and nitric acid HNO 3 etc. Chemical calculators Downloads Prices. 3 Link Save Widget.
15 August 1994 OSHA. Arrhenius Definition of Acids and Bases. PH of a solution calculator.
3 a 1 b 1 c 3 d Li. Cesium hydroxide CaOH 2. Salicylic Acid is a type of beta hydroxy acid BHA and phenolic acid with a chemical formula C 7 H 6 O 3.
URL copied to clipboard. Strong bases are the hydroxides of the alkali Group IA and alkaline earth Group IIA metals ions which are sufficiently soluble. It is a BHA found as a natural compound in plants.
In fact this is only one possible set of definitions. Formulas of Ternary Acids. H2S04 H Br LiOH KOH HN03 Define the following terms.
Why LiOH is a strong base. LANRAN help customers in lithium extraction and conversion separation and purification of life sciences chemical and petrochemical system resource recycling silicon and semiconductor clean. Circle the salt produced in each re action.
The other bases make. Weak acids do not have this ability. 0a 1 b 0c.
PH of a strong acidbase solution. HN03 I can label an acid as mono di 8. 2 a 1 b 2 c 0d S.
LiOH Lithium hydroxide Li-aq OHaq. A compound is a weak base when it partially or not completely dissociate in an aqueous solution which means not all moles of the base. LiOH or tri protic.
Ion exchange membrane consumption of single project. Calcium hydroxide SrOH 2. Consider the titration of 200 mL of 0100 M perchloric acid with 0400 M LiOH.
A strong acid or a strong base completely ionizes dissociates in a solution. WB490000 NaOH and basic salts 5611 KOH 1310-58-3 TT2100000 KOH 2395 LiOH 1310-65-2 OJ6307070 LiOH METHOD. Acid is a substance that ionizes in water to give H ions.
C 2 mgm315 min NaOH. H 2 SO 4. HCCOH HClO4 HI NH3 NaOH HNO2 CH3COOH CH32NH HNO3 CaOH2 HCl KOH HCN HF CH3NH2 CsOH HBr LiOH BaOH2 H2SO4.
LiOH lithium hydroxide NaOH sodium hydroxide KOH potassium hydroxide CaOH 2 calcium hydroxide RbOH rubidium hydroxide SrOH 2 strontium hydroxide CsOH cesium hydroxide BaOH 2 barium hydroxide Did you know that fluorantimonic acid is an example of a very strong acid known as a super acid. Answer Key Ionic Molecular or an Acid Honors Chemistry Write which type of compound it is whether the compound is ionic molecular or an acid. 000 250 500 750 1000 and 1100 of equivalence.
2 Changing the colour of litmus from red to blue. PH calculator program - Base Acid Titration and Equilibria - dissociation constants pKa and pKb. Amphoteric indicators nee A reaction between an.
C 2 H 5 NH 2 N. Also Read-Why NaOH is a strong base. H2SO3 LiOH H2O Li2SO3 1.
Barium hydroxide BaOH 2 Calcium hydroxide CaOH 2 Lithium hydroxide LiOH Sodium hydroxide NaOH etc. Conjugate acids cations of strong bases are ineffective bases. Disclaimer - accuracy of the values shown especially for the strong acids is.
The name of the most common form of the acid consists of the nonmetal root name with the -ic ending. Acid Acid is something. Label Each Compound With a Variable a H 2 SO 3 b LiOH c H 2 O d Li 2 SO 3 2.
If there is a multi-valent metal present write both the stock and classical name. Enter your answers without units. Definitions of Acids and Bases.
B Base as 1 Being slippery and. And the compound such as NaOH KOH LiOH BaOH 2 etc. It functions as a plant hormone.
CHEMISTRY 1A NOMENCLATURE WORKSHEET Chemical Formula Nomenclature Practice. C 2 mgm3 NaOH PROPERTIES. This lipophilic monohydroxybenzoic acid is a derivative of salicin metabolism.
Although the general properties of acids and bases have been known for more than a thousand years the definitions of acid and base have changed. 7401 Issue 2 EVALUATION. Complete these in lab and on your own time for practice.
Label the following as mono di or triprotic. Complete and balance the following reactions. What is the pOH when 50 L of a 045 M solution of sulfuric acid H 2 SO 4 is titrated with 23 L of a 12 M lithium hydroxide LiOH solution.
It is extremely corrosive and must. The acid containing one less oxygen atom than the most common form is designated by the -ous ending. It is poisonous when.
HClO 4 perchloric acid List of Strong Bases 8. The term strong in the name refers to the acids ability to release hydrogen H molecules which allows it to become ionized when placed into a solution of water. CaOH2 KOH AlOH3 LiOH Write formulas for the following bases.
NaOH KOH LiOH MW. Digits after the decimal point. Chemical compound molar mass.
Calculate the pH at. About 11 10000 square meters. Group I metal hydroxides LiOH NaOH etc Group II metal hydroxides MgOH2 BaOH2 etc Strong bases completely dissociate in aq solution Kb 1 pKb 1.
We can define acids as substances that dissolve in water to produce H ions whereas bases are defined as substances that dissolve in water to produce OH ions. To solve this problem we must first determine the moles of H ions produced by the strong acid and the moles of OH-ions produced by the strong base respectively. Why BaOH 2 is a strong base.
The pKa values for organic acids can be found in. An acid containing one less oxygen atom than the -ous acid has the prefix hypo-and the -ous. 44 Perchloric acid H-aq ClO aq H 24 SO Sulfuric acid 2 H-aq SO 4 2aq b Strong bases.
Calculators used by this calculator. CH 3 NH 2. 1 a 0b 0c 1 d O.
Hydrochloric acid is prepared by dissolving gaseous hydrogen chloride in water. Boric acid is determined by ashing tissues in an alkaline medium at 600 C dissolving in hydrochloric acid centrifuging mixing part of the supernatant with carminic acid in sulfuric acid and measuring the color at 575 nm after 1 hr.

Coke Cans In Acid And Base Periodic Table Of Videos Youtube
John Wiley and Sons 1991-Present p.
Sulfuric acid dissolving metal. How can sodium hypochlorite be produced. Citric acid is a weak acid found in citrus fruits and used as a natural preservative and to impart a sour flavoring. Precipitation of AgCl.
At 145C the stream of steam is increased and during 25-3 hours the phenylacetone is steam. Purpose of Electroplating. Because of the corrosive nature of the acid ceramic glass or sometimes tantalum apparatus is commonly used.
The solution is electrolyzed and forms a sodium hypochlorite. Filter with vacuum assist and let vacuo suck as dry as possible. The dissolved metal ions are reduced at the cathode plating the metal onto the item.
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride commonly known as concentrated hydrochloric acid. Lysergic acid and potassium sulphate will be seen to precipitate. Reacting copperII oxide with sulfuric acid.
1025g 75 sulfuric acid was mixed with 1g ZnCl 2 and 192g 116 mol Ephedrine or Pseudoephedrine freebase was dissolved at a temperature of 50-100C and the reaction mixture was heated further to 145-150C. The acid creates a layer on the steel and this layer prevents the acid from dissolving the metal. Less harmful gasses are produced when sulfuric acid is used.
To the filtrate add a solution of 25 g 12-tungstosilicic acid in 20 cm 3 water slowly with stirring. Nitric acid HNO 3 is a strong acid because when it is dissolved in an aqueous solution it completely dissociates into H and NO 3 ions in the solution. Carbonic acid is a weak acid.
HNO3 H2O -. Sodium hypochlorite can be produced in two ways. At 125C steam is passed through the solution to facilitate mixing of the contents.
Includes kit list and safety instructions. As on dissolving HNO 3 in an aqueous solution no hydrogen remains bound all are dissociated and converted into H ions which means the concentration of hydrogen ion increased in the solution. In association with Nuffield Foundation.
Hydrofluoric acid is actually a weak non-oxidising acid and in the good old days it was often used to dissolve mud and sand that had clogged-up the cooling water passages of marine. 93 wt sulfuric acid has a freezing point below zero at -21ᵒF so many industrial applications can utilize carbon steel tanks with 93 wt H 2 SO 4 uninsulated but 98 wt freezes at 30ᵒF and so much more care is needed for successful storage. Atoms are represented as spheres and are colour-coded.
Although nearly 100 sulfuric acid solutions can be made the subsequent loss of SO 3 at the boiling point brings the concentration to 983 acid. Stir the solution for a few minutes and filter off any precipitate. Laboratory chemicals Synthesis of substances 13 Details of the supplier of the safety data sheet Company.
For example concentrated sulfuric acid often referred to as Oleum is normally transported in steel tanker wagons. Let stand for 2-3 hours in the 5-10 cooling mixture. It is used to.
The remaining liquid is known as raffinate a waste product. LOUIS MO 63103 UNITED STATES. Simply the acids concentration becomes diluted.
Make a solution from 150 ml of liquid ammonia and 25 liters. In soils these acids can cause the release of natural but toxic elements like aluminum. It is used in the dye industry as a mordant and as a reducing agent.
Sulfuric acid is a strong acid that strongly reacts with bases and that is very corrosive. HClg in water produces hydrochloric acid which is a solution of hydronium and chloride ions. And when these compounds react with water vapor in the atmosphere they turn into sulfuric acid and nitric acid and then return to the surface as acid rain.
The freezing point of different sulfuric acid concentrations can vary markedly. FeCl 2 4H 2 O by dissolving metallic iron in hydrochloric acid. However there is an acid base reaction that Nitric acid is constantly undergoing which is.
This is the only acid excreted by the lungs as a gas. Ferric chloride is generally prepared from ferrous chloride through the action of chloride or nitric acid. B the appearance of a substance changes but its identity does not.
Dissolve 05 g of ferrocene in 10 cm 3 concentrated sulfuric acid. Now Is HNO 3 Nitric acid strong or weak. 3050 SPRUCE ST ST.
Direct current is supplied to the anode oxidizing its metal atoms and dissolving them in the electrolyte solution. - By dissolving salt in softened water which results in a concentrated brine solution. Sulfuric acid can be obtained by dissolving sulfur trioxide in water.
Allow the solution to stand for at least half an hour then pour it into 150 cm 3 distilled water. With a little dilute sulfuric acid. Answer 1 of 11.
The current through the circuit is such that the rate at which the anode is dissolved is equal to the rate at which the cathode is plated. Solutions of hydrocyanic acid and potassium nitrite are mixed. NO 2 1 nitrite ion.
Aqua regia dissolves gold although neither constituent acid will do so aloneNitric acid is a powerful oxidizer which will actually dissolve a virtually undetectable amount of gold forming gold ions Au 3The hydrochloric acid provides a ready supply of chloride ions Cl which react with the gold ions to produce tetrachloroaurateIII anions also in solution. It is responsible for dissolving limestone to produce geological features such as stalagmites and stalactites. Physical properties Grades of sulfuric acid.
H 2 SO 4aq HSO 4-aq H aq The second dissociation constant is much smaller than the first so the reaction in which HSO 4-loses a proton H does not go to completion the ions are in equilibrium with the undissociated acid molecules. Ferric sulfate is produced on a large scale by adding sulfuric acid and an oxidizing agent eg nitric acid or hydrogen peroxide. Between the sulfuric acid concentrations of 35 - 75 wt freezing is.
H ions are attracted to the cathode gain electrons. Sulfuric acid Product Number. Illustrate the reaction of an insoluble metal oxide with a dilute acid to produce crystals of a soluble salt in this class practical.
Break up the filter cake and put in a 2 liter beaker. In water they can poison aquatic wild life and on land the acidity can cause animals eggs to not hatch and plants to lose nutrients. Sulfuric acid is a polyprotic strong acid The first dissociation constant is very large so that we assume this reaction goes to completion.
We identify HCN as the reacting acid K 1 as a spectator ion and the NO 2 1 ion as. 7664-93-9 12 Relevant identified uses of the substance or mixture and uses advised against Identified uses. Neutralize the mixture by adding cold dilute sulfuric acid to a congo red end point pH 42.
Anhydrous liquid hydrogen chloride is. There are several reasons. And form hydrogen gas.
Another process called electrowinning uses electricity to extract copper out of the PLS onto thin metal sheets. V2 289 1992 Hazardous Substances Data Bank HSDB Made commercially from aluminous materials such as bauxite. Then the copper-bearing liquid is combined with another acid to precipitate the copper from the organic material.
The 983 grade is more stable in storage and is the usual form of what is described as concentrated sulfuric acid. Now things have. Nonahydrate Kirk-Othmer Encyclopedia of Chemical Technology.
If water is acidified. Metal Ions as Lewis Acids. Raffinate can contain concentrated amounts of TENORM.
Prepared by dissolving aluminum or aluminum hydroxide in dilute nitric acid and crystallizing the product from the resulting aqueous solution. OH-ions are attracted to the anode lose electrons and form. B crushing the sugar cube and dissolving it in water c dehydrating the sugar cube with sulfuric acid d chewing the sugar cube and digesting it 9 A physical change occurs when a both the appearance of a substance and its identity change.