Sodium hydroxide Na OH also known as lye or caustic soda is a caustic metallic base. The G-value was automatically calculated by the program-controlled jar test apparatus ZR4-6 Zhongrun Water Industry Technology Development Co.
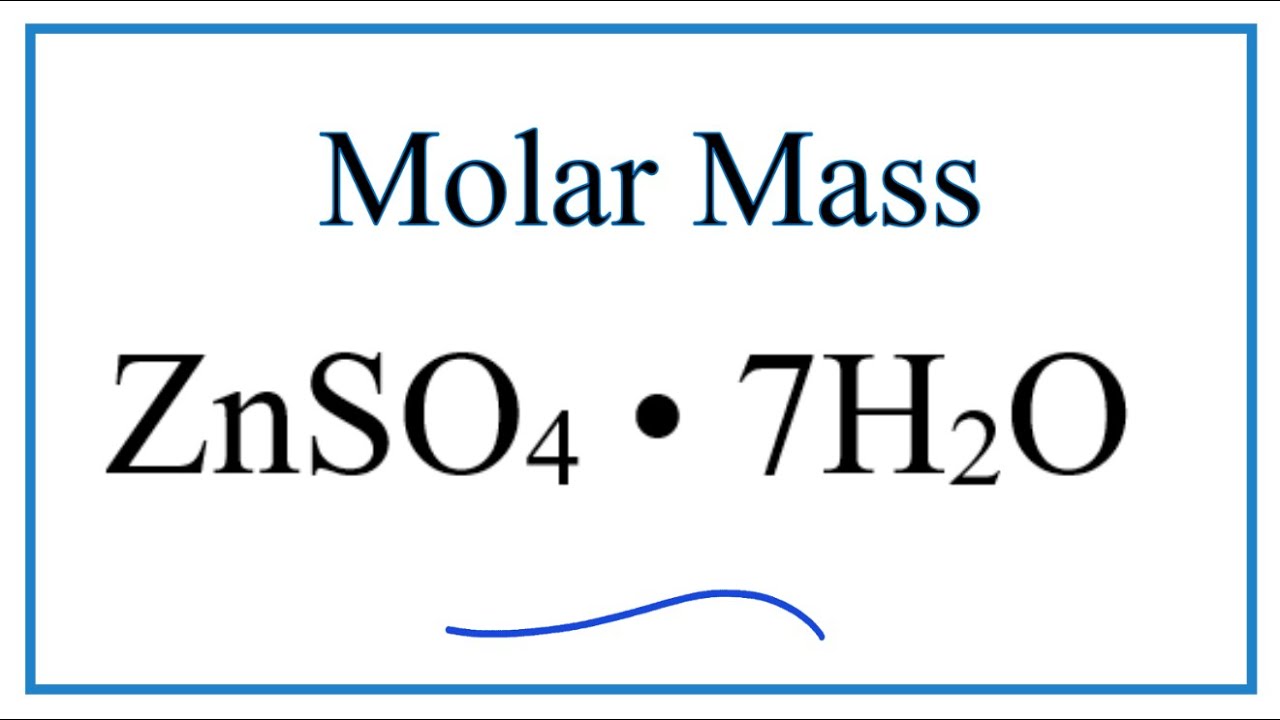
Molar Mass Molecular Weight Of Znso4 7h2o Zinc Sulfate Heptahydrate Youtube
104 gmol 1302 gmol 799 or 8.

Zinc silicate molar mass. Mole concept molar mass and percentage composition. 8 will serve as a multiplier MOLECULAR FORMULA IS C 8H 8 357 Hydrofluoric acid HFaq cannot be stored in glass bottles because compounds called silicates in the glass are attacked by the HFaq. Heavy metals as Pb 00005.
Cu Copper 00002. Al Aluminium 0001. Na Sodium 05.
An introduction to atomic number isotopes and isobars. Molar mass M is the mass in grams of one mole of a chemical substance. Variations of molar conductivity.
Sulfate SO₄ 00005. Difference between atom and ion. Atomic mass molecular mass.
SiFe molar ratio of 14. Determine the mass of gold that can be extracted if 250 g of sodium cyanide is used. The vapor pressure of pure water at this temperature is 238.
Hafnium dioxide is an intermediate in some processes that give hafnium metal. Molar heat capacity. Atomic mass unit amu a unit of mass equal to 112 the mass of the carbon isotope with mass number 12 approximately 16604 x 10E-24 gram.
The present study measured strontium Sr isotopes with hydro-geochemistry data of 56 water samples in detail in the MRB in northeast Thailand. Molar Mass of Gas. View Answer When does the.
Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. Calcium sulfate or calcium sulphate is the inorganic compound with the formula CaSO 4 and related hydratesIn the form of γ-anhydrite the anhydrous form it is used as a desiccantOne particular hydrate is better known as plaster of Paris and another occurs naturally as the mineral gypsumIt has many uses in industry. Zinc silicate powder ZS was mixed with multi-walled carbon nanotubes MWCNTs at various mass fractions 0 1 2 and 3 wt followed by argon sintering.
X-ray diffraction XRD analysis. Thereafter the mass ratios of the four. Atomic mass the mass of an atom expressed in atomic mass units amu.
For example the molar mass of carbon is 12 g mol1. As the largest and most representative tributary of the Mekong River the Mun River Basin MRB provides critical understanding of regional hydro-geochemical features and rock weathering processes on a basin scale. Atomic mass and molecular mass.
269815385 7 Standard state. That is while the fractional mass ratio of the asphaltenes remained the same at 10 the rest of the components decreased with aging ie from 27 to 10 for saturates 31 to 10 for aromatics and 39 to 11 resins. Worldwide production in 1998 was around 45 million tonnes.
Reaction mass of 2-chloroethyl chloropropyl 2-chloroethylphosphonate mixture reaction mass of isomers and 2-chloroethyl chloropropyl 2-chloropropylphosphonate reaction mass of isomers 401-740-0 015-144-00-X reaction mass of pentyl methylphosphinate and 2-methylbutyl methylphosphinate 402-090-0 87025-52-3 015-145-00-5. All forms are white solids that are poorly soluble in water. For monatomic ideal gases the ratio of those at constant pressure and volume is 53.
Collecting Gases Over Water. Daltons Law of Partial Pressure. Cyclohexanone-formaldehyde resin produced when 1 mole of cyclohexanone is made to react with 165 moles of formaldehyde such that the finished resin has an average molecular weight of 600-610 as determined by ASTM method D2503-82 Standard Test Method for Molecular Weight Relative Molecular Mass of Hydrocarbons by Thermoelectric Measurement of Vapor Pressure which is incorporated by.
Pb Lead 00005. Relative atomic mass A r. Total nitrogen N 00005.
93347 66032 C 122058 F K. Gas Diffusion and Effusion. Therefore the molar mass of an element or compound is the relative atomic molecular or formula mass amount expressed in grams.
Sodium hydroxide is also the most common base used in chemical laboratories. An alkali caustic soda is widely used in many industries mostly as a strong chemical base in the manufacture of pulp and paper textiles drinking water and detergents. Van der Waals Equation.
Zinc-free floor finish water-based styrene acrylic polymer emulsion for high traffic applications such as schools hospitals and retailMor-Glo G128 is recommended for low maintenance floor finishes. 5611 gmol Pricing Availability. Van der waals forces.
Ni Nickel 00005. What quantity for ten points is defined as the heat capacity per mass. Determine the mass of glucose molar mass 180 gmol needed to add to 5000 g of water to change the vapor pressure to 231 torr.
The oxygen in these oxides and in other compounds mostly silicate minerals and calcium carbonate in limestone makes up nearly half of the Earths crust by mass. The Effect of Intermolecular Forces. Besides the hexagonal wurtzite structure of ZnO expands its volume around 16 times higher than the metallic zinc and hence its deposition on the anode surfaces may cause.
Sodium silicate Na 2SiO 3 for. Many molecules in living things have oxygen in them such as proteins nucleic acids carbohydrates and fats. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
Solid at 298 K. The Effect of the Finite Volume. Since CH has a formula weight of 1302 divide molar mass by that.
Most living things use oxygen in respiration. In the coagulation tests we just needed to set the G value and the program. P is the rate of energy dissipation per unit mass of water W m 3 and μ is the dynamic viscosity of water Pa s.
The molar mass of an element or compound is given the unit grams per mole or g mol1. Silicate SiO₂ 0005. For water at 15 degrees Celsius it is defined as 1 calorie per gram per degree Celsius.
4Au 8NaCN O_2 to 4NaAuCN_2 4NaOH. Ca Calcium 0001. Atomic molar mass gmol Symbol Electronegativity Atomic number Key iron 26 5585 Fe 18 3 2 europium 63 15196 Eu 3 2 americium 95 243 Am 3 4 gadolinium 64 15725 Gd 12 3 curium 96 247 Cm 3 terbium 65 15893 Tb 3 berkelium 97.
235 W m 1 K 1. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. A higher molar volume in comparison with zinc metal due to its hexagonal wurtzite structure is the main reason for drastic effects of the deposited layers of ZnO on the anode surface from the structural point of view.
It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. Difference between elements and atoms. It can be easily formulated into EPA Safer Choice Green Seal and Ecologo certifiable floor.
Distribution of Molecular Speeds and Collision Frequency. The total number of protons and neutrons in the nucleus. 2792 2519 C 4566 F K.
150 g of NaCl completely dissolves producing Na and Cl ions in 100 kg of water at 250 C. HafniumIV oxide is the inorganic compound with the formula HfO 2Also known as hafnium dioxide or hafnia this colourless solid is one of the most common and stable compounds of hafniumIt is an electrical insulator with a band gap of 5357 eV. Kinetic Molecular Theory and Gas Laws.
The vapor pressure of water at 250 C is 238 torr. Oxygen is a part of water which all known life. One form is the partial derivative of the enthalpy per unit mass with respect to temperature taken at constant pressure.
Atomic number the number of protons in the nucleus of an atom. Fe Iron 00005.
In the example provided above it can be observed that the coordination number of the central cobalt atom is 6 since it is bonded to 6 different nitrogen atoms. The molar mass of the NaOH compound is 40 gmol.

Cobalt Element In Periodic Table Atomic Number Atomic Mass
Investigating Molecular Structure - The Link Between Size and MW.
Molecular mass of cobalt. Lastly we emphasize that its molecular structure is critical and contains the active site even if support effects are important. Amedeo Avogadro is considered to be the number of units usually molecules or atoms in a substance that is proportional to its physical mass. A12 protons 12 neutrons.
The tabular chart we see on the periodic table is arranged. A student wants to create a model of a cobalt atom. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
98101106107109 c At constant Al level polymer molecular weight MW increases with the Clalkyl ratio in the aluminum alkyl chloride. The alkene called but-2-ene has two isomers which the chemical formula CH 3 CHCHCH. Some of these.
This mass proportion will be the same for any water molecule. Here we report a cobalt-phthalocyanine-based high-performance carbon dioxide reduction electrocatalyst material developed with a combined nanoscale and molecular approach. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structureThe chemical symbol for Hydrogen is H.
Which statement about the model is correct. Of or relating to chemistry. It has the mass composition of.
Enter a chemical formula. Molecular formulae indicate the simple numbers of each type of. Use the periodic table to determine which atom would have similar chemical properties to this atom.
Now molecular mass calculator add all masses of substances together. What is the molecular formula of a compound that has a molecular mass of 54 and the empirical formula C_2H_3. In a periodic table there are a total of 118 elements.
We would like to show you a description here but the site wont allow us. The atomic mass is the mass of an atom. Cobalt-60 a radioactive isotope of cobalt is an important source of gamma rays and is used to treat some forms of cancer and as a medical tracer.
In such cases the value of the coordination number of the central atom equals the total number of neighbouring. The polymer molecular weight increases with the monomer concentration 148099101106301 Figure 3 while it decreases with increasing cobalt concentration. Cobalt blue is an important part of artists palette and is used bu craft workers in porcelain pottery stained glass tiles and enamel jewellery.
Cyclohexanone is produced by the oxidation of cyclohexane in air typically using cobalt catalysts. To Determine the Molecular Formula. Which statement about the model is correct.
Of or relating to chemical weapons. Atomic mass is also referred to as atomic weight but the term mass is more accurate For instance it can be determined experimentally that neon consists of three isotopes. In related terms another unit of mass often used is Dalton Da or unified atomic mass unit u when describing atomic masses and molecular masses.
The molar mass of a compound is 11938 g mol 1. Molecular weight calculator is an online tool to calculate atomic mass and molecular mass. It is possible to determine molecular size and MW distributions for a polymer using GPCSEC and the aforementioned measurement methods.
Did you mean to find the molecular weight of one of these similar formulas. More information on molar mass and molecular. This compound is a cobalt complex.
An atom has 3 protons 4 neutrons and 3 electrons. The explanation of structural characteristics depends on the use of these data along with. The atomic mass is carried by the atomic nucleus which occupies only about 10-12 of the total volume of the atom or less but it contains all the.
Other cobalt macrocycle complexes such as cobalt chlorin and. Cobalt has a mass number of 59 and an atomic number of 27. On the nanoscale cobalt.
Atomic Mass of Cobalt. Neon-20 with 10 protons and 10 neutrons in its. Cobalt has a mass number of 59 and an atomic number of 27.
Cobalt chloride hexahydrate is a hydrate of cobalt chloride containing cobalt in 2 oxidation state chloride and water moieties in the ratio 126. The molecular weight calculator uses the molar mass of each element in the formula you specify to determine the molecular weight of the total compound. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
With a standard atomic weight of circa 1008 hydrogen is the lightest element on the periodic table. How many elements are in the periodic table. The radioactive isotopes cobalt-60 is used in medical treatment and also to irradiate food in order to preserve the food and protect the consumer.
Molecular size like MW is a distributed parameter. Use the periodic table to determine which atom would have similar chemical properties to this atom. 100000 Similar chemical formulas.
Cobalt-60 has a half-life of 527 years and decays into nickel-60 through beta decay. This process co-forms cyclohexanol and this mixture called KA Oil for ketone-alcohol oil is the main feedstock for the production of adipic acid. Molecular mass or molar mass are used in stoichiometry calculations in chemistry.
Also important in this field is Avogadros number N. An atom has 3 protons 4 neutrons and 3 electrons. A12 protons 12 neutrons.
The molar mass of the compound is unknown. A student wants to create a model of a cobalt atom. Of or relating to the properties or actions of chemicals.
Cobalt compounds have been used for centuries to color porcelain glass pottery tile and enamel. It has a role as. Chemical names in answer to limitations of chemical formulae.
CO2 Co2 Calculate the molecular weight of a chemical compound. It has the mass composition of 678 of hydrogen 3142 of nitrogen 3976 of chlorine and 2204 of cobalt. A drug especially an illicit or addictive one.
We see that 94 of the mass of a water molecule is accounted for by oxygen and the remaining 6 is the mass of hydrogen. Question abc31 Cobalt a transition metal can have an oxidation number of either 2 or 3. C 6 H 12 O 2 CH 2 5 CO H 2 O.
The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. Atomic mass of Cobalt is 589332 u. A substance with a distinct molecular composition that is produced by or used in a chemical process.
16 g mol 23 g mol 1 g mol 16 g mol 40 g mol 23 g mol 1 g mol 40 g mole. Molecular mass calculator helps a user to complete his work in a given time without any errors. For crystals the bonds are not as clear in their solid state structures.
The atomic mass is a weighted average of all of the isotopes of that element in which the mass of each isotope is multiplied by the abundance of that particular isotope. 80102104 The effect of the concentration of Al seems to depend on the type of aluminum. Note that all formulas are case-sensitive.
Its monatomic form H is the most abundant chemical substance in the Universe constituting roughly 75 of all baryonic mass. It is defined to be 112 of the mass of one atom of carbon-12 and in older works is also abbreviated as amu. The units used are grams per mole because the molecular weight is usually expressed as the mass of one mole out of a certain substance.
Browse the list of common chemical compounds.
How many grams of NaCl are required to prepare 985 mL of 077 M NaCl solution. Dichlorine - Cl 2.

Find The Molecular Mass Of Mgso4 7 H2o Brainly In
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4.
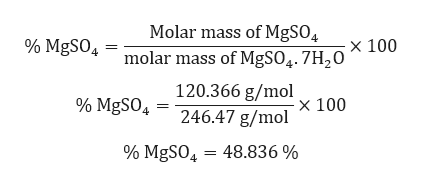
Mgso4 molar mass. MgSO_4 7H_2O The idea here is that heating the hydrate will drive off the water of evaporation and leave behind the anhydrous salt. MgSO47H2O molecular weight. 5 H2O CuOH2 H2SO4 K2Cr2O7 NaCl CaC4H4O6 MgCrO4 Al2SO43 K3PO4 ZnCl2 COMPOUND FORMULA MASS CuSO4.
C mass of water mass of hydrate mass of anhydrate 6923 g 3382 g 3541 g H2O d Calculate molar masses of the components of the hydrate. 24305 32065 1599944 71007942 159994 Percent composition by element. Blamp Created Date.
A few things to consider when finding the molar mass for HClO4- make sure you have the c Perchloric Acid HClO4 Molar Mass Molecular Weight. 52 gram per cubic centimeter or 2 520 kilogram per cubic meter i. MgSO4 where MM MgSO4 the molar mass of MgSO 4 Moles of water lost M 2 W 3MM H2O where MM H2O the molar mass of H 2O Ratio of moles M 2M 1 Do division and report first number with 2 significant digits.
Metal carbonates are weak bases. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry. Heat and Chemical Changes 42.
Magnesium is a Group 2 alkaline earth element within the periodic table and has a relative atomic mass of 24305 Da a specific gravity at 20C of 1738 2 3 a melting point of 6488C and a boiling point of 1090C. Molar mass of MgSO4 1203676 gmol. Formula Mass 1641 MgSO4.
Masas Moleculares Calculadas Recientemente. Divide the weight of the compound by the compounds molar mass measured in grams to obtain the number of moles. 892007 101757 AM.
Scacchite - MnCl 2. This compound is also known as Magnesium Sulfate. To determine the percentage of morphine in the white powder the mass of morphine reacted was divided by the total mass of the white sample 0008094g0010g 100 810.
What is the molarity of an aqueous solution of sodium hydroxide produced when 350 ml of a 540 M solution was diluted to 8900 ml. 0450 kg of octane C8H18 burned during combustion produces 125 kg of carbon. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems.
Water - H 2 O. 0063432 gmol Molar mass of MgSO4 is 120. CHEM 232 Organic Chemistry for Sodium chloride ˌsoʊdiəm ˈklɔːraɪd commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions.
Magnesium sulfate or magnesium sulphate in British English is a chemical compound a salt with the formula MgSO 4 consisting of magnesium cations Mg 2 2019 by mass and sulfate anions SO 2 4It is a white crystalline solid soluble in water but not in ethanol. You can also ask for help in our forums. Use the molecular formula to calculate the molar mass.
Element Family Reunion 51. When you put sodium carbonate Na2CO3 in water it produces 2 sodium ions and one carbonate ion. Thus the hydrated magnesium cation is hard to dehydrate.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Lea nuestro artículo sobre cómo calcular la masa molar. Potassium Sulfate - K 2 SO 4.
What is the theoretical yield of a reaction if the percent yield 924 and product recovered is 893 g. Which of the following is a strong acid. Academiaedu is a platform for academics to share research papers.
In the dissolved state magnesium binds hydration water tighter than calcium potassium and sodium. MnCl2 Molar Mass MnCl2 Oxidation Number. Convert grams MgSO4 to moles or moles MgSO4 to grams.
Convert grams MgSO47H2O to moles or moles MgSO47H2O to grams. Mystery Anions 70. Hydrogen Chloride - HCl.
Magnesium sulfate is usually encountered in the form of a hydrate MgSO 4 nH 2 O for various values of n between 1 and 11. With molar masses of 22. The Colligative Properties of Solutions 39.
Subsequently question is how do you find the moles of water in hydrated MgSO4. What does that mean regarding the metal ions that make up the carbonate salt. Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number.
Organizing Topic Electron Configuration and the Periodic Table 49. 24305 32065 1599944 Percent composition by element. K2So4 Kaliumsulfat Potassium Sulphate Dipotassium Sulfate K2SO4 Molar Mass K2SO4 Oxidation Number.
They react with acids to release carbon dioxide. 1 Show answers Another question on Chemistry. Microsoft Word - HYDRATESdoc Author.
C2H52O Ether NH42C2O4 Ammonium Oxalate NH42CO3 Ammonium Carbonate NH42CrO4 Ammonium Chromate NH42HPO4 Di-Ammonium Phosphate NH42S Ammonium Sulfide NH42SO4 Ammonium Sulfate NH43PO3 Ammonium Phosphite NH43PO4 Ammonium Phosphate Ag2O SilverI Oxide Ag2S Silver Sulfide Ag2SO4 Silver. Cl₂ Chlorine Gas Bond Polarity. If the molar mass of the salt is 218 gmol what mass is required.
Molar Heat of Fusion for Water 36. Organizing Topic Bonding Nomenclature and Formula Writing 59. Calculadora de Estequiometría de las.
A Crystal Lab 61. Atomic Mass of Atoms. If you use 0001 gl as your concentration and desire to convert say 250 ppm Magnesium sulfate to molar concentration you could actually multiply the ppm concentration by 0001 gl then divide.
What is the mass percent of MgCO33H2O in the mixture. Report second number as 1 Title. 최근 계산한 몰 질량.
In your case the anhydrous salt is magnesium sulfate MgSO_4. H2O CaOH2 AlCl3 D. Round atomic masses to the tenth of a decimal place.
Hcl HCl Molar Mass Bond Polarity HCl Oxidation Number. Water - H 2 O. 664 gcm³ 1.
Molecular Model Building 65. When CaOH2 reacts with H2SO4 what are the products. Balanceo de ecuaciones químicas.
How many grams of H_3PO_4. 7 H2O Cu2C4H4O6 CoClO32 BeCr2O7 KBr FeNO33 HgSO4 Ca3PO42 NiSO3 AgNO2 COMPOUND FORMULA MASS. For example if the atomic mass of sulfer S is 32.
Since you know that after complete dehydration the mass of the sample is equal to 482 g you can say that the hydrate contained 482 g - MgSO_4 Use the molar mass of the compound to. Koh Lye E525 Potash Lye Caustic Potash Potassium Hydrate KOH Molar Mass KOH Oxidation Number. Molar Mass of Frequently Calculated Chemicals.
MgSO4 1204 gmol and H2O 1802 gmol e Calculate the moles of MgSO4 in the hydrate. The mass of morphine reacted was found by multiplying the moles reacted by the molar mass of morphine 0008094 g. 113 10-2 moles.
The amount of metal ion in solution stays the ph changes. Molar mass of MgSO47H2O 24647456 gmol. Find the formula mass of the following compounds.
Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. What volume of 12 M HCl solution is needed to prepare 5 liters of 00250 M solution. Mno2 Mno₂ ManganeseIv Oxide MnO2 Molar Mass MnO2 Oxidation Number.
화학량론 화학 반응 계산기. To explore more about the structure physical and chemical properties of Magnesium sulfate MgSO 4 from the experts register with BYJUS now. How many moles are in 125 g of calcium chloride.
In the nuclear industry chlorine trifluoride is used to prepare uranium hexafluoride a volatile compound of uranium used in the separation of uranium isotopes. Example Exercise 91 Atomic Mass and Avogadros Number.
Elemental nitrogen is a colorless odorless tasteless and mostly inert diatomic gas at standard conditions constituting 7808 by volume of Earths atmosphere.
Molecular mass of nitrogen trifluoride. Properties of Nitrogen trifluoride. Molecular geometry of NF3. The mass of Avogadros number of atoms is the atomic mass expressed in grams.
Cu 6355 amu Hg 20059 amu S 3207 amu and He 400 amu. It finds increasing use within the manufacturing of flat-panel displays photovoltaics LEDs and other microelectronics. It is Corrosive to tissue.
Some boron compounds such as boron nitrite are completely water insoluble. Write the Lewis structure for a molecule of the compound. It is possible to draw a structure with a double bond between a boron atom and a fluorine atom in BF 3 satisfying the octet rule but experimental evidence indicates the bond lengths are closer to that expected for.
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. Boron salts are generally well water soluble. If a gas behaves ideally both a and b are zero.
We would like to show you a description here but the site wont allow us. Nitrogen trifluoride or NF3 is a nitrogen halide compound that is slightly water-soluble. Briefly 4 mL of 05 M sodium hydroxide in methanol were added to 1 mL oil sample the equipment was closed and heated for 20 min under nitrogen.
The reac-tion was evaporated in vacuo quenched with water 40 mL adjusted to pH 9 with Na 2 CO 3 extracted with CHCl 3 2 x 100 mL dried over anhydrous Na 2 SO 4 filtered and evaporated in vacuo to a semi-crystalline mass. It is usually referred to as the van der Waals equation of state. Further 5 mL of 15 boron trifluoride freshly prepared.
For example in the Lewis structures of beryllium dihydride BeH 2 and boron trifluoride BF 3 the beryllium and boron atoms each have only four and six electrons respectively. Van der Waals suggested a modification to take into account molecular size and molecular interaction forces. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab.
Chlorine trifluoride is prepared by the reaction latextextCl_2g3textF_2glongrightarrow2textClF_3glatex. A compound with a molar mass of about 42 gmol contains 857 carbon and 143 hydrogen by mass. Atoms of a Cu 6355 g c S 3207 g b Hg 20059 g d.
Its refractive index is 10004. The atomic mass of each element is listed below the symbol of the element in the periodic table. Nitrogen trifluoride NF 3 is an inorganic colorless non-flammable toxic gas with a slightly musty odor.
The human body contains about 3 nitrogen by mass the fourth most abundant element in the body after oxygen carbon. The constants a and b are called van der Waals constants. They have positive values and are characteristic of the individual gas.
Learn about Lewis Acids and Bases Examples Applications Reactions and FAQs Visit BYJUS for detailed explanations. Lewis Acids and Bases -Lewis Acids are the chemical species which have empty orbitals and are able to accept electron pairs from Lewis bases. Two arrangements of atoms are possible for a compound with a molar mass of about 45 gmol that contains 522 C 131 H and 347 O by mass.
Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight or molar mass can be calculated by adding the weight of each component. Flask containing MeOH 50 mL and boron trifluoride diethyl etherate 10 g 705 mmol and refluxed for 2 hours. What is the empirical formula of a compound that contains 494 K 203 S and 303 O by mass.
Atomic or molecular chemical species having a highly localized HOMO The Highest Occupied Molecular Orbital act as Lewis bases. P anV 2 Vn - b RT. Boron trifluoride is the least water soluble boron compound with a water solubility of 24 gL.
This method with usage of acid catalyst boron trifluoride is supported by the fact that basic catalysis does not convert free fatty acids especially in oils as can be concluded from the paper. It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. Many olefin polymerization reactions use BF3 as an initiator in conjunction with a proton donor such as.
Boron trifluoride is commonly used as a catalyst for Friedel-Crafts alkylation reactions. At a temperature of 25 degrees Celsius the solubility of boric acid in. NF3 has a molar mass of around 71002 gmol and a density of 3003 kgm3.
Here we present a modified version of the Medusa. D K2SO3 Lithium and nitrogen react in a combination reaction to produce lithium nitride. It has a low dipole moment.
Nitrogen trifluoride NF 3 first prepared in 1928 is a colourless and odourless gas that is thermodynamically stable and most readily produced by the electrolysis of molten ammonium fluoride dissolved in anhydrous hydrogen fluoride. Write the equation that relates the rate expressions for this reaction in terms of the. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
Write the Lewis structures for the two molecules. The density of NF3 is 3003 kgm 3. Nitrogen trifluoride is also an extremely strong and long-lived greenhouse gasIts atmospheric burden exceeded 2 parts per trillion during 2019 and has doubled every.
Air - Thermophysical Properties - Thermal properties of air at different temperatures - density viscosity critical temperature and pressure triple point enthalpi and entropi thermal conductivity and diffusivity. Nitrogen ˈ n aɪ t r ɵ dʒ ɨ n ny-trə-jin is a chemical element that has the symbol N atomic number of 7 and atomic mass 1400674 u. It has a molar mass of 7100 gmol.
Why is boron present in water. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. The solubility of H 3 BO 3 in water is temperature-dependent.
This potent greenhouse gas has a rising atmospheric abundance due to its emission from a growing number of manufacturing processes and an expanding end-use market. Like carbon tetrafluoride it is not. NF3 boiling point is 12906 C and melting point is 20715 C.
Electron geometry of. Under standard conditions for temperature and pressure STP boric acid exists as a white crystalline solid that is fairly soluble in water. Its noticeable characteristics include being colorless and carrying a musty or moldy odor.
It also is used to catalyze the cleavage of ethers to alcohols to catalyze esterification reactions and in the nitration and sulfonation of aromatic compounds. Boric acid has a water solubility of 57 gL borax of 252 gL and boron trioxide of 22 gL. We present an analytical method for the in situ measurement of atmospheric nitrogen trifluoride NF3 an anthropogenic gas with a 100-year global warming potential of over 16000.
Boron Trifluoride 330 Bromine Pentafluoride 670 Bromine Trifluoride 670 1-3 Butadiene 510 Butane 510 Butenes 510 Carbon Dioxide 320 Carbon Monoxide 350 Carbonyl Fluoride 660 Carbonyl Sulfide 330 Chlorine 660 Chlorine Trifluoride 670 Chlorotrifluoroethylene 660 Cyanogen 660 Cyclopropane 510 Deuterium 350 11-Difluoroethylene 350. Molecular Weight Molar Mass.
This material isnt. Problem 7 A 100 mathrmkg sample of magnesium at 400circ mathrmC is added to 100 mathrmL of water maintained at 200circ mathrmC in an insulated container.

Chemical Composition By Mass Of Quicklime And Slaked Lime Wt Download Scientific Diagram
When used in this way it absorbs carbon dioxide.

Molar mass of quicklime. Substancial - Free ebook download as Text File txt PDF File pdf or read book online for free. What are its units. 10 22 atoms of an element X are found to have a mass of 930 mg.
Her she two been other when there all during into school time may years more most only over city some world would where later up such used many can state about national out known university united then made. N The number of greenhouse gases emitted. What can be the molar shape of BCl3.
5162F 2850C Soluble in. Note that the increase in the unit weight and the reduction in the moisture content are two important factors in stabilization of the closed. Calcium oxide CaO commonly known as quicklime or burnt lime is a widely used chemical compound.
The reducing agent or reductant is an element that loses or donates electrons ie. How precise is this method of mass determination. Dilute the compound with.
This yields our molar mass of 97995181 or 98 gmol. Quicklime is an oxide that when bright into contact with an acid forms salts. The formula of this chemical substance is C24H30N2O3 and the molar mass equals 394.
Calcium oxide also known as quicklime is an alkaline substance that has been in use since the medieval age. 5 For purpose of determining if an emission threshold has been exceeded include in the emissions calculation any CO 2 that is captured for transfer off site. The mass of a proton or neutron is approximately _____ times that of an electron.
Calculate the molar mass of the element X. Weigh out the necessary amount of compound in grams and set it aside. The molar mass of the compound is 182 gmol.
In this case H2Mo04 acts as an oxidising agent. This method is often referred to as the slaking of lime. Hardness of diamond is due mainly to a large amount of A.
GHG i Mass emissions of each greenhouse gas metric tonsyear. Define atomic mass unit. Units are indicated with graphical representations that suggest a schematic physical design of the unit.
Steps 1 and 2 can be used to convert stack gas concentrations from mgdscm to a Cl- So for a buffer strength of 0. What is the reducing agent. Weigh out the mass of the compound.
Had first one their its new after but who not they have. It can also be referred to as burnt lime or lime. Selected gas and liquid streams show the most important constituents using mass fraction as for gaseous streams and molar concentration for aqueous.
It is not found in a free state in nature but is found commonly as NaCl solid or seawater. Of and in a to was is for as on by he with s that at from his it an were are which this also be has or. Molar mass of an element is the mass of Avogadros number of atoms.
It is believed that quicklime is one of the oldest chemicals known to the human race. The water temperature rises to 554circ mathrmC What is the mass of the steel. The normal calcium oxide-associated molar entropy corresponds to 40 joules per mole kelvin.
The mass of one mole of a substance is called its molar mass. Department of Health and Human Services Food and Drug Administration CHEMICAL CO. The other is l-methamphetamine which makes the heart race but does little to the brain.
1007943 30973761 1599944. It is waste product. It forms slaked lime when it interacts with water.
The equivalent mass of Na2 S 2 0 3 in its reaction with I2 is molar mass divided by two. Files can be shared directly between systems on the network without the need of a central server. The atomic mass of hydrogen is 100794 oxygens is 159994 and that of phosphorus is 30973761.
It is a white caustic alkaline crystalline solid at room temperature. An element consist of 6010 of an isotope with an atomic mass of 68926 amu and 3990 of an. Northern Arizona University and Raymond Chang this success guide is written for use with General Chemistry.
Calcium oxide has a medium viscosity and a high surface tension plus a high to intermediate expansion and contraction rate. Referring to Table 86 with regard to the revised compaction tests by mixing MSW samples with 267 fly ashquicklime the maximum dry density increased from 1132 kgm 3 to 1182 kgm 3 and the optimum moisture content decreased from 157 to 133. What is molar mass.
View Answer Calculate the volume of a gas sample at T -150 degrees C and P 494 mmHg if the same sample occupied 500 L at T 200 degrees C and P 798 mmHg. Chang General Chemistry The Essential Concepts 6th txtbkPDF. Quicklime is known to crystallize in a cubic crystal lattice.
What will be the final. GWP i Global warming potential for each greenhouse gas from Table A-1 of this subpart. The broadly used term lime connotes calcium-containing inorganic materials in which carbonates oxides and hydroxides of calcium silicon magnesium aluminium and iron predominate.
Using a calibrated balance place a weighing dish and zero it out. One atomic mass unit is a mass unit equal to exactly one twelfth 112th the mass of one atom of carbon -12. Once you have calculated the desired mass you need to weigh it out.
In linear polymers the individual polymer chains rarely have exactly the same degree of polymerization and molar mass and there is always a distribution around an average value. The relative atomic mass of oxygen atom is 16. Mixed phase streams with substantial solid-phase mass flow are color-coded based on the phase of the gas or liquid transporting the solid.
It allows the chemist to determine the amount of product that will form from a given amount of reactants or the amount of one reactant that is needed to react completely with some specific amount of the other reactant. The atomic mass is multiplied by the number of atoms present in phosphoric acid. Its unit is gram per mole g mol -1.
C To calculate GHG emissions for. Chemical dosing calculator excel. It is used as a wall coating like a whitewash.
Calculate the empirical and molecular formulas. The molar mass distribution or molecular weight distribution describes the relationship between the number of moles of each polymer species N i and the molar mass M i of that species. Find the molar mass of the gas.
Contains some random words for machine learning natural language processing. The most common area that hydrated lime is used in is the building industry. Always clean the balance of any powder before continuing to make the solution.
Hence the computation follows. Stoichiometry is the chemistry that mathematically relates all substances in a reaction quantitatively relating the amount of reactants and products in a chemical reaction. Weigh out 25 g of NaCl.
When 1125 g of a liquid hydrocarbon C_xH_y was burned 3447 g CO_2 and 1647 g H_2O. How To Use Hydrated Lime On The Homestead. Determination of the reducing and oxidizing agents is coming in 2022.
6022 x 10 23 atoms will have mass frac09301022 x 6022 x 10 23 g 560 g Molar mass of the element 56 g mol-1. It aims to help students hone their analytical and problem-solving skills by presenting detailed approaches to solving chemical problems. There are three hydrogen atoms one phosphorus atom and four oxygen atoms.
In a reaction H2Mo04 is changed to Mo0 2. UNK the.