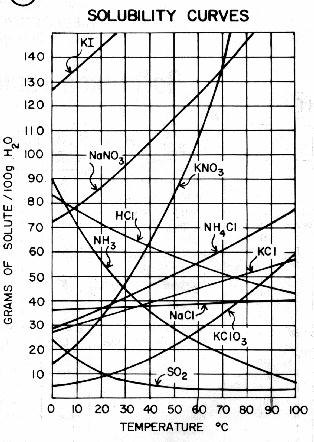
00002500 mol NaOH x 1000 mL 001618 M NaOH 1545 mLNaOH 1 L. This means it has a pH toward the top end of the pH scale which ranges from 0 to 14.

Uses Of Sodium Hydroxide Assignment Point
Sodium hydroxide is easy to handle inexpensive and very effective for the neutralization of strong or weak acids.

What is the use of naoh. Municipal water treatment facilities use sodium hydroxide to control water acidity and to help remove heavy metals from water. When using scientific notation use it correctly do not use the WebCT or Excel way of writing scientific notation. ACID BASE TITRATION Concentration of NaOH 01 MC Trial 1 Trial 2 Trial 3 Trial 4 Initial buret reading 9 mL 6 mL 8 mL 6 mL Final buret reading 265 mL 266 mL 255 mL 256 Volume of NaOH added 2565 26 247 25 Moles of NaOH added Moles of HCl reacted Molarity of diluted HCI Molarity of undiluted HCI Average molarity of undiluted HCI Precision 0513.
For example sodium hydroxide NaOH a base and hydrochloric acid HCl give sodium chloridecommon saltand water through the reaction NaOH HCl- NaCl HOH H2O. Here the chemical group formula is. What is the percentage of KHP in this sample.
Calculate the molar concentration of the sodium hydroxide solution using your data for each run. Practical report - Titration of hydrochloric acid with Sodium Hydroxide Caution. Calculate the average molar concentration of the sodium hydroxide solution.
Preparation of KHP Acid. This is called standardization of the solution. Sodium hydroxide NaOH also known as caustic soda or lye is a highly versatile substance used in a variety of manufacturing processes.
The same color changes happen with the next three trials. The concentration of the 1 M NaOH solution provided to you will not be known accurately so you will measure the concentration of a diluted solution by using it to titrate a well-known amount of acid. NaOH is a strong alkali and HCl acid is a strong acid respectively.
Calculating the limiting reactant the change in enthalpy of the reaction H rxn can be determined since the reaction was conducted under conditions of constant pressure H rxn q rxn moles of limiting reactant. Note 2 Other methods are discussed in the textbook. From the mole ratio the number of moles of NaOH 000979 mol.
Hydrochloric acid as well as Sodium Hydroxide are both very strong acidbase and harmful to skin and eyes. If any contact to the human body would occur that section of the body needs to be washed thoroughly with a good amount of water and taken to the emergency room if necessary. In the storage of liquid NaOH the ability to maintain the solutions temperature and correlating viscosity is generally considered a requirement of large-scale store and use.
Concentration of NaOH was. Mole ratio 1 KHP1NaOH. For example it is 2500 x 10 4 mol NaOH not 2500E-4 mol NaOH.
Note that rounding errors may occur so always check the results. 491M 1975 385 Belongs to the Following Reactive Groups Bases Strong. To aid in temperature maintenance containers equipped with heat tracing and insulation are recommended for many applications.
Molecular weight of NaOH or mol This compound is also known as Sodium Hydroxide. As demonstrated in the freezing point curve graph for sodium hydroxide at various. Use this page to learn how to convert between grams NaOH and mole.
Weight of weighing boat before adding KHP 267 g. Use this page to learn how to convert between moles NaOH and gram. 1 grams NaOH is equal to 0025001806380511 mole.
Also known simply as caustic is the most widely used alkaline neutralizing chemical in use in industry today. This reaction is classified as an. Didnt Read Using a pH indicator strip will tell you that NaOH sodium hydroxide is a strong alkaline.
Liquids with this reactive group classification have been known to react with the absorbents listed below. Thats because NaOH cant be bought chemically pure and because its so hygroscopic that its mass will visibly increase while it is being weighed. NaOH is clear and transparent solution with no color.
Sodium Hydroxide NaOH Sodium Hydroxide NaOH. You can see a white precipitate is deposited at the bottom of the solution when two solutions are mixed. Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily.
In the first trial after adding 90 drops of NaOH solution there was repeatedly appearance and disappearance of light pink color. Hot andor concentrated NaOH can cause hydroquinone to decompose exothermically at elevated temperature. HClaq NaOHaq -- NaClaq H 2 Ol Energy.
C NaOH nV 00097900950 0103 mol dm-3 cm 3 is converted into dm 3 Raw Data. Step pH ApH use step 3 as initial pH Buffer pH step 3 Procedure 3 Use this for ApH calcs 912 NA this is the initial 913 915 926 Buffer pH after adding 100 PL NaOH step 4 Buffer pH after adding 200 PL NaOH step 5 Buffer pH after adding 300 PL NaOH step 5 Buffer pH after adding 400 ML NaOH step 5 Buffer pH after adding 500 ML NaOH. Identify combustion through unique reactantproduct features.
Calcium chloride CaCl 2 reacts with aqueous NaOH to produce calcium hydroxide CaOH 2 and NaClCaOH 2 is a white precipitate. A pH testing strip will tell you that NaOH sodium hydroxide is a strong alkaline but to calculate its exact pH you have to work out its molarity first. Unless the expected.
Sodium hydroxide is also used to produce sodium hypochlorite a water disinfectant. STOPPER AND SAVE YOUR NaOH SOLUTION FOR USE IN EXPERIMENT 12B. The titration of an impure sample of KHP found that 360 mL 0100 M NaOH was required to react completely with 0765 g of sample.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Sodium Hydroxide in Food Production. Answer 1 of 4.
When the whole solution of KHP and water get titrated then the color of solution becomes light pink and it stays permanently. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. To calculate the exact pH work.
Thermochemistry determine the heat exchanged at constant pressure q m c T. Weight of weighing boat with. 1 mole is equal to 1 moles NaOH or 3999711 grams.
The SI base unit for amount of substance is the mole. To identify the equivalence point in the titration we use titration curves and indicatorsAccording to the concentration of acid and base solutions we have to choose correct curve and indicator. Note that rounding errors may occur so always check the results.
Sodium hydroxide NaOH or HNaO CID 14798 - structure chemical names physical and chemical properties classification patents literature biological activities. A Na B OH C Cl D H. In other words you have DeltaH_diss - 6322 J Convert the mass of sodium hydroxide to moles by using the compounds molar mass 24 10-4 colorredcancelcolorblackg 1 mole NaOH39997colorredcancelcolorblackg 600 10-6 moles NaOH You know that the enthalpy of dissolution when 600 10-6 moles of sodium hydroxide are dissolved in water so use.
CaCl 2 NaOH CaOH 2 NaCl Reaction and Characteristics. Potentially Incompatible Absorbents. KHC 8 H 4 O 4 NaOH H 2 O NaKC 8 H 4 O 4.
NaOH is available in concentrations of up to 50 which is the most commonly used concentration. In practice the concentration of an NaOH solution is never determined by calculating it from mass and volume. If you need to prepare roughly one liter of 1 M NaOH solution you dissolve the molar mass of NaOH 400 g using distilled water in a beaker then transfer this solution to a one liter volumetric.
Carbonate-free solutions can be obtained simply by diluting 50 NaOH at the time of use. THIS IS THE VALUE THAT YOU WILL USE IN EXPERIMENT 12B. Water and Aqueous Solutions.
Prepare approximately 250 mL of CO2-free water by boiling on a hot plate for 5 minutes. K 2 O immediately converts to KOH when water is added.
/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)
How To Prepare A Sodium Hydroxide Or Naoh Solution
Balanced reaction of CaCl 2 and NaOH with physical states CaCl 2aq NaOH aq CaOH 2s 2NaCl aq CaCl 2 is very much soluble in water.
Naoh in water. But solubility of CaOH 2 is low in water and may depend on the ion concentrations of Ca 2 ions OH-. Set the boiling water well back from the bench edge to avoid accidents and burns. Generally this is a safe and inexpensive base to use for the neutralization of acidic materials.
230 M e Calculate the volume of a 350 M NaOH solution that must be added to 500 mL of water to produce 100 M NaOH. Acetil salicylic acid is an organic acid having one carboxilic group which confers the acidity to the acetil salicylic acid and then we can write it as C8H7COOH then a neutralization reaction of the acid with soda NaOH will be C8H7COOH NaOH C8H7COONa H2O or C9H8O4 Na. Reducing the pH will shift the balance toward the hypochlorous acid.
When water dissociates it yields a hydrogen ion and a hydroxide. Therefore reactions using NaOH will not normally generate high solids unlike calcium products lime or magnesium products magnesium hydroxide. Clean your 250 ml Erlenmeyer flask and rinse it with distilled water.
Incorrect The solution is 50 Molarity. D Calculate the molarity of a KOH solution if adding 35 mL of it to 65 mL of water produced 0800 M KOH. Theoretical titration curve of glycine with NaOH.
Sodium salts are normally quite soluble in water. Back to Neutralization Chemicals. Water and NaOH solutions can absorb carbon dioxide CO2 gas from the air which will react with water to form carbonic acid H2CO3.
You will use your data to determine the pK a and pI values of each amino acid thereby allowing you to deduce the identity of your unknown and those. Acetic acid - diluted solution. The 6M NaOH reagent bottle is on your bench.
Next drain about 5 mL of the solution through the tip to rinse and fill the tip with the solution. With the NaOH solution twice with approximately 10-mL portions. Molarity M-is the molar concentration of a solution measured in moles of solute per liter of solution.
Sodium Hydroxide in Food Production. CH 3 COOH NaOH CH 3 COONa H 2 O Check the balance Acetic acid react with sodium hydroxide to produce sodium acetate and water. 125 molal NaOH 125 mole NaOH 1 kg H 2 O 125 mole NaOH x 40 g NaOH 500 g NaOH 1 mole NaOH Measure 500 g NaOH and add water to 1 L volume.
Be sure to ALWAYS USE UNITS. In this video well balance the equation Na H2O NaOH H2 and provide the correct coefficients for each compoundTo balance Na H2O NaOH H2 youll n. In practice the concentration of an NaOH solution is never determined by calculating it from mass and volume.
If you are given concentration in any other unit than moles mass percent molality etc convert it to molarity in order to use the pH. Solid NaOH has a melting point and boiling point of approximately 604F and 2534F. 375 mL x 00750 28125 mL ethylene glycol 28125 mL ethylene glycol x 109 g ethylene glycol1ml 307 g ethylene.
Study TECH sections. In 1000 g of NaOH there are 25 moles of NaOH. Citation needed In that pH range.
First thing is there is no K 2 O solution. Thermodynamic properties of substances The solubility of the substances Periodic table of elements. 52 g K 2 SO 4 x 1 mole K 2 SO 4 0299 mole K 2 SO 4 174 g K 2 SO 4 0299 mole K 2 SO 4 0073 M 4100 L K 2 SO 4 6.
Therefore it exists as an aqueous solution and NaOH also exists as a solution. Aqueous aq water solution. Sodium hydroxide is also used to produce sodium hypochlorite a water disinfectant.
These compounds ionize in water to yield one or more hydroxide ion OH- per molecule of base. 200 mL f Calculate the volume of water that must be added to 15 mL of 0600 M HCl to make 0100 M HCl. The rinse solution should go into the waste beaker.
Add 01 ml of 01 molar NaOH to 50 ml of 01 molar potassium hydrogen phthalate. During an acid-base titration there is a point when the number of moles of acid H ions. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH.
Take 125 ml of above stock solution and bring. In this video we will describe the equation NaOH H2O and write what happens when NaOH is dissolved in waterWhen NaOH is dissolved in H2O water it will d. For pH 400.
Correct The solution is 50 Molar. Fill the buret to the very top. Bring volume to 1 liter with distilled water.
Meaure between 8 and 85 ml of 6M NaOH using a clean but not necessarily dry 10 ml graduate cylinder. At 25 0 C temperature solubility of NaOH is 1000 g for one liter of water. 2500 mL HCl x 1 L x 00100 mol HCl x 1 mol NaOH 00002500 mol NaOH 1000 mL 1 L 1 mol HCl When using scientific notation use it correctly do not use the WebCT or Excel way of writing scientific.
Add 291 ml of 01 molar NaOH to 50 ml 01 molar potassium dihydrogen phosphate. The molarity definition is based on the volume of the solution NOT the volume of water. Moles NaOH Molarity NaOH Volume NaOH NoteCalculations can be done in steps or in a single step as long as your TA can easily follow what you are doing.
Sodium hydroxide NaOH also known as caustic soda or lye is a highly versatile substance used in a variety of manufacturing processes. BC 367 Experiment 1 Fall 2009 3 Experimental Procedure You will perform automated titrations of both a known standard 01 M glycine and an unknown amino acid also at 01 M with 10 M NaOH. If you tilt the.
H 2 O H OH-When calculating pH remember that refers to molarity M. Dissolve 8954g of disodium hydrogen phosphste12 H 2 O and 3. Municipal water treatment facilities use sodium hydroxide to control water acidity and to help remove heavy metals from water.
Therefore 10M naOH solutions can be exist. At a pH between 55 and 60 approximately 90 of the ions are in the form of hypochlorous acid. Adjust pH to 72 with 1 N NaOH.
See this chemical equation below. Thats because NaOH cant be bought chemically pure and because its so hygroscopic that its mass will visibly increase while it is being weighed. HClaq NaOHaq NaClaq H 2 Ol heat.
Because KOH and HCl are strong base and. Ammonia is a good example of a weak base. Unless the expected.
Sodium hydroxide is less soluble in polar solvents such as methanol but is readily soluble in water up to a 50 wt solution which will have a pH around 14. The chemical solution of 50 NaOH is denser than water with a density around 152gcm 3 at 68F. Answer 1 of 4.
In contrast a weak base only partially dissociates into its ions in water. Sodium hydroxide - concentrated solution. Pour the NaOH solution into the 250 ml Erlenmeyer flask and then add distilled water.
Since the solutions are mostly water the solutions are assumed to have a density of 10 gmL and a specific heat of 418 JgC. A solution whose pH is 73 will contain equal concentrations of hypochlorous acid and hypochlorite ion. Answer 1 of 3.
Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. What is the ph range when a titration of K2O solution with HCl reaches equivalence point. Sterilize 15 min at 121C.
PO Box 95 716 Visions Drive. The reaction of an aqueous hydrochloric acid solution with an aqueous sodium hydroxide solution is represented by the neutralization chemical equation. Dissolve 120g of sodium dihydrogen phosphate and 0885g of disidium hydrogen phosphate in 1 liter volume distilled water.
Allow the water to cool in a beaker covered with a watchglass. It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Base to produce salt and water.
Molarity is expressed in units of moles of solute per liter of solution. Electrolysed water electrolyzed water EOW ECA electrolyzed oxidizing water.
From the equation 2 mol of NaOH reacts with 1 mol of Na 2 SO 4 so 05 mol of NaOH will react with 025 mol of Na 2 SO 4. HCl aq NaOH aq NaCl aq H 2 O l acid base salt water.
Chapter 6 Chemical Composition 2006 Prentice Hall Chapter
How does temperature affect solutions.
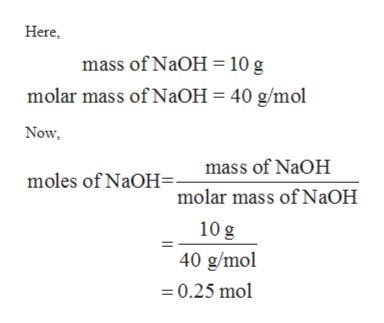
Mass of naoh. If the equation is arranged correctly the mass units g cancel out and leave moles as the unit. Now let us consider the solution obtained by mixing. This compound is also known as Sodium Hydroxide.
How are solution solute and solvent related. B Why in step 2 should the pipet and beaker be rinsed with. Write the equation for the titration.
Calculate the moles n of NaOH. G HCl 50. Of moles of KHP Mass of KHP used Molar mass 042 g 20422 gmol 00021 moles 2.
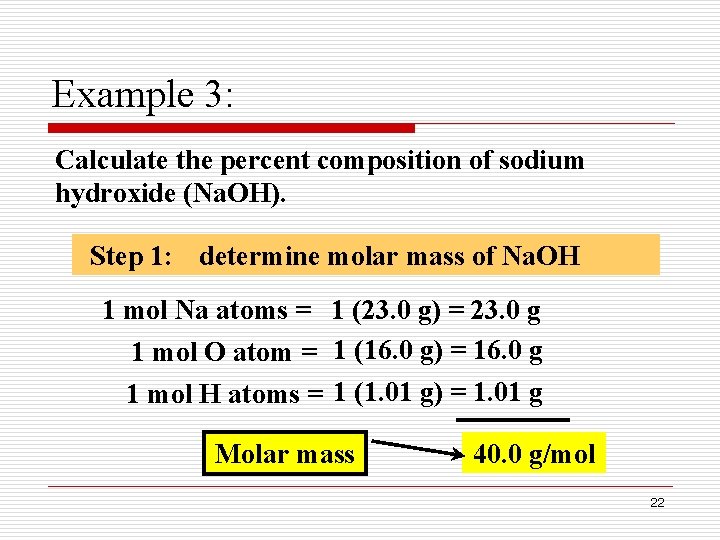
Uses the formula of a reactant to determine molar mass. Molar Mass Molar mass Mass in grams of one mole of any element numerically equal to its atomic weight Molar mass of molecules can be determined from the chemical formula and molar masses of elements Each H 2O molecule contains 2 H atoms and 1 O atom Each mole of H 2O molecules contains 2 moles of H and 1 mole of O One mole of O atoms corresponds to 159994 g Two moles of H atoms. The concentration of NaOH is 400 g L 1.
In other words you have DeltaH_diss - 6322 J Convert the mass of sodium hydroxide to moles by using the compounds molar mass 24 10-4 colorredcancelcolorblackg 1 mole NaOH39997colorredcancelcolorblackg 600 10-6 moles NaOH You know that the enthalpy of dissolution when 600 10-6 moles of sodium hydroxide are dissolved in water so use. Grams of NaOH 301 x 10-3 x 6005 molar mass 018075 grams Grams of CH3COOH Grams of NaOH at equivalence point the point where the solution was neutralized Step 4. The molarity of the dilute NaOH solution is not determined from the volumes of the concentrated NaOH solution and water added together.
Answer 1 of 4. Molar Mass of NaOH sodium hydroxide Molar Weight Calculator Our Other Math. A 718 b 319 c 598 d 144 e 239 all x 1023 3.
It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. How does a solution differ from a colloid. Check the chart for more details.
Standardization of a Sodium Hydroxide solution NaOH Sample Code O Trial 1 Mass of KHP transferred 042 g Volume of Distilled water 25 mL Volume of NaOH used 2250 mL Molar mass of KHP 20422 gmol No. Q solution 50. If you need to prepare roughly one liter of 1 M NaOH solution you dissolve the molar mass of NaOH 400 g using distilled water in a beaker then transfer this solution to a one liter volumetric.
Two moles of NaOH will be left unreacted. What mass of sodium hydroxide is needed to react completely with 100 g of ironIII chloride. Total mass of solute W B 30 g 60 g 90 g.
A hot water solution containing 731 mass of NaOH is an eutectic that solidifies at about 6263 C as an intimate mix of anhydrous and monohydrate crystals. It is an intensive property. If you have dissolved 1 g of NaOH in enough water to make a total of 250 ml of solution calculate the number of moles of solute present by diving the mass of NaOH by the molecular mass of the compound.
2 Al 6 NaOH ----- 2 Na3AlO3 3 H2 What mass in grams of Na3AlO3 is produced when 6 x 1023 molecules of NaOH is consumed. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. Molar mass of NaOH 3999711 gmol.
Visit BYJUS for more information. Latex90text gspace textNaOH times frac1 text mol40text g 225 space textmol NaOH. Number of moles of NaOH mass relative formula mass 20 40 05 mol.
Its formula is. On the other hand the molarity of KHP solution cNaOH cKHP is determined based on the mass of the KHP and the volume of water it is dissolved in. The average NaOH titre was 3212 mL Calculate the mass of calcium carbonate in grams present in the chalk sample.
A second stable eutectic composition is 454 mass of NaOH that solidifies at about 49 C into a mixture of. In practice the concentration of an NaOH solution is never determined by calculating it from mass and volume. Mass of solute in this solution 30 of 100 g 30100 x 100 g 30 g.
It is denoted by ρ i. But it may vary with pressure and temperature. Do a quick conversion.
G NaOH418 Jg C400C - 200 C 8360 J. Make sure that you count the atoms for each element. Instead it is determined via the KHP titration.
Find the molar mass of each element using the periodic table of elements. 2298977 159994 100794 Percent composition by element. Find the molar mass of the solute.
The energy released by the reaction is q reaction. Consider 150 g of a 40 solution of NaOH. This can be done by adding together the separate molar masses of each element found in the solution.
This the same as multiplying by the reciprocal of 40 gmol. Total mass of solution W A 100 g 150 g 250 g. If three moles of FeCl 3 and three moles of NaOH are brought together two moles of FeCl 3 will remain unreacted.
Amedeo Avogadro is considered to be the number of units usually molecules or atoms in a substance that is proportional to its physical mass. A 240 b 80 c 64 d 96 e 48 2 Given the balanced equation. The molecular mass of NaOH is 40 so work out 1 40 0025.
Atomic Mass of Atoms. Determine the amount of HCl in excess from the titration results. CuCl 2 CuCl2 C 12 H 22 O 11 C12H22O11 C 6 H 5 3 PCCO.
And thus mass of NaOH moles xx molar mass 00513cancelmolxx400gcancelmol-1 205g. Mass of solute in this solution 40 of 150 g 40100 x 150 g 60 g. Unless the expected.
By the law of conservation of energy. The excess HCl was then titrated with 0250 mol L-1 NaOH. To calculate the number of moles from the mass or grams of solute used you must first determine the molar mass of the solute.
NaOH are brought together only three moles of the NaOH will react. NaOH HOOC-C6H4-COOK Æ NaOOC-C6H4-COOK H2O By measuring the volume of the 02M NaOH solution dispensed from the buret that is necessary to react completely with a weighed sample of KHP the exact concentration of NaOH solution is calculated. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
The intake of AlOH 3 is 500 mg. Thats because NaOH cant be bought chemically pure and because its so hygroscopic that its mass will visibly increase while it is being weighed. The SI unit is kg m 3.
Convert grams NaOH to moles or moles NaOH to grams. Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH. Enter formulas with proper capitalization and unpack brackets.
Check out a buret from the stockroom. The mass concentration of H 2 SO 4 and SO 4 is 392 g L 1 and 384 g L 1. 3 CuS 8 HNO3 ----- 3 CuNO32 3 S 2 NO 4 H2O What number of molecules of CuNO32 is produced when 67 g of HNO3 is consumed.
The reaction between NaOH and KHP molar mass 20423 gmole is as follows. 1 moles NaOH 3999711 gram using the molecular weight calculator and the molar mass of NaOH. Q solution m c T where m is the total mass of the resultant solution c is the specific heat capacity of the solution and T T f -T i.
Why do solutions become saturated. The molecular weight of sodium hydroxide is 40 gmol. Mass concentration is the mass of a solute per unit volume of the solution.
Amadeo Avogadro first proposed that at a given temperature and pressure the volume of a gas is proportional to the number of atoms or molecules and has nothing to do with the type. The first step is calculating the number of moles of solute present. The molar mass of the NaOH compound is 40 gmol.
Hydrogen chloride and hydrogen cyanide Tips Acids are always aqueous. 1990-1998 Associate Professor Iowa State University 1988-1990 Associate Professor of Chemistry and Director of Freshman.
The potential does vary with temperature but between 10 40C can be estimated by the equations see reference 2.
Naoh solubility vs temperature. The solvent that has a high solubility of solute and low solubility of carrier liquid is the ideal solvent that must be chosen in liquid- liquid extraction process. Where T is the temperature C and E. 1998-2013 Professor of Chemistry Iowa State University.
The solubility of benzoic acid in water at 95 oC is 68 gL. By signing up youll get. If the DS is 250 ml but in the range of 250 to 1000 ml in.
Leave the thermometer in the water as you go on to the next step. The pK a for acetic acid is 476. This is consistent with the weakly basic character of LiOH in solution indicating that the Li.
With the increase in temperature stripping efficiency increases. To remove soluble impurities. Temperature is one of the most important parameters in the fermentation and stripping processes.
50 cal cm312. In maceration whole or boorishly powdered plant material is kept in contact with the solvent in a stoppered container for a given period with repeated agitation until soluble matter is dissolved at room temperature for a period of 3 days Handa et al 2008. Example of K a vs.
The process has the purpose to soften and break the plants cell wall to release the soluble phytochemicals. Any phrases that refer to being dissolved or in solution means the compound is aqueous. NaOH CO2 ----- NaHCO3 156 D.
The K a for acetic acid is 175 10 5. The synthesis of polyolefin DRAs is based on low-temperature ZieglerNatta ZN polymerization of higher α-olefins. It is therefore necessary to adjust the stripping temperature to.
LiOH however has a layered structure made up of tetrahedral Li OH 4 and OHLi 4 units. PRECIPITATIVE GRAVIMETRIC ANALYSIS Precipitative gravimetric analysis requires that the substance to be weighed be readily removed by filtration. 047 0015 cal g C.
Viscosity cP at 25C See Figure 4 20. In this article we will explain the electronic configurations ionization enthalpy hydration enthalpy and atomic ionic radii and other physical and chemical properties of the group one alkali metals. Ultra-high molecular weight poly-α-olefins are widely used as drag reducing agents DRAs for pipeline transportation of oil and refined petroleum products.
E 205 073 T 25 for an electrolyte of 35 M KCl E 199 101 T 25 for an electrolyte of saturated KCl. Note that the relation between the solubility and the solubility product constant depends on the stoichiometry of the dissolution reaction. Measure the temperature of the warm water in the Styrofoam cup and record this value in the data table.
RECRYSTALLIZATION Briefly describe how soluble impurities are separated from the desired compound at the molecular level. 4353 dynes cm. 130 cal cm312.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Vapor pressure at 25C See Figure 1 0600 mm Hg. What is the minimum amount of water in which one gram of benzoic acid can be dissolved at 95 oC.
Stronger acid larger K a smaller pK a. Magnesium hydroxide MgOH 2 is. Immediately after recording the temperature add the equivalent of a handful of ice cubes to the warm water.
Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Show all calculations for full credit. The solubility in water of the other hydroxides in this group increases with increasing atomic number.
The room-temperature form of NaOH has the thallium iodide structure. 2015present Senior Instructor II University of Oregon. Solubility in Chemistry refers to the ability of a substance to be combined with another substance.
Surface tension at 20C. 2018 compared NaOH KOH and LiOH solution respectively at a concentration of 7 wv under the temperature ranging between 8C and 20C for rice straw pretreatment the highest lignin removal of 6322 was observed after LiOH pretreated at 15C for 3 min. 80 ca l cm312 Hydrogen bonding.
Elements hydrogen nitrogen oxygen fluorine chlorine ammonia carbon monoxide and carbon dioxide nitrogen monoxide and nitrogen dioxide sulfur dioxide and sulfur trioxide hydrogen-compounds eg. RECRYSTALLIZATION Draw a solubility y vs temperature x plot showing the three common solubility behaviors and indicate which one is that of a good recrystallization solvent. At room temperature 25 oC the solubility of benzoic acid in water is 34 gL.
Do not allow any. The mixture then is. The relationship is below.
For this reason it is meaningless to compare the solubilities of two salts having the formulas A 2 B and AB 2 say on the basis of their K s values. The use of. 90 cal cm312 Polar.
Whereas a DS 250 ml in aqueous media indicates solubility issues in the corresponding GI segments a DS 250 ml at all pH values of interest indicates that dissolution is very unlikely to limit drug absorption. High solubility at high temps and low solubility at low temps. Weaker acid smaller K a larger pK a.
The important thing to note is that the pK a scale is opposite that of the K a scale. Learn its full definition its properties and the different factors that affect solubility. Room-temperature acetylene electroreduction then occurs at a layered double hydroxide LDH-derived copper electrocatalyst which offers an onset potential of.
2013-2014 Visiting Lecturer University of Oregon. 20132015 Morrill Professor Iowa State University. Alkali metals belong to the s-block elements occupying the leftmost side of the periodic tableAlkali metals readily lose electrons making them count among the most reactive elements on earth.
Typically the deposition was performed in a standard two-electrode configuration at room temperature with an electrolyte of 20 M NH 4 Cl and 01 M NiSO 4. Looking at the table at the top of this article the. If you have 155 mL of a 0762 M FeCl3 solution how many grams of FeCl3 are contained in this sample.
Department of Geology and Geophysics University of Utah Salt Lake City Utah 84112 USA. The pK a value is more convenient. Thus the solubility is 88 times 105.
2006 Visiting Professor University of Arizona. 1-Hexene based DRAs the most effective at room temperature typically lose DR activity at low temperatures. Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete Marie D.
Be very careful not to add any cold water melted ice in the 400-mL beaker to the warm water. The highest ethanol extraction efficiency of 964 was at 75 C but this temperature has a negative effect on microorganism growth and increases the energy costs. Which of the following ions always form soluble ionic compounds_ select all that apply.
Subsequent to the solubility experiments the dosesolubility ratio DS should be calculated according to the BCS. Specific heat at 295C. In order for a non-filtrable precipitate to form it must be supersaturated with respect to its solubility product constant.
Search for other works by this author on. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. In dilute solution at equilibrium the concentration of.
Besides the solvent which nonreactive with the other chemical involved in the extraction and has high boiling temperature are suitable for liquid-liquid extraction process.
00 amu 3 The molecular mass of H2SO4 98. Na O H NaOHa O H NaOH.

Answered What Mass Of Sodium Hydroxide Naoh Bartleby
It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps.

Naoh molecular mass. A second stable eutectic composition is 454 mass of NaOH that solidifies at about 49 C into a mixture of crystals of the dihydrate and of the 35-hydrate. Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH. Next calculate the number of liters of solution present.
Atomic Mass of Atoms. Molecules of CO2 48g Molarity dilutions. Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.
Assuming your 20 is weight on weight just add 80 grams. To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. What is the molecular weight of an unknown monoprotic acid if 04955 g of the acid are neutralized by 3700 mL of a 01000 M NaOH solution.
The molecular weight of the substance and the identity of the unknown can than be determined based on the number of moles and the weight of the sample. Our molar mass calculator uses the. Work out 250 1000 025.
Convert to liters by dividing by 1000 because there are 1000 milliliters in one liter. Of any pure substance has a mass in grams exactly equal to that substances atomic or molecular mass mass of one mole of a substance. TextEquivalent Mass of NaOHdfrac40140 _square Equivalent Mass of NaOH 1 4 0 4 0.
The molar mass of the NaOH compound is 40 gmol. Molecular weightmolar mass of MgCl 2. 1 u is equal to 112 the mass of one atom of carbon-12.
Latex90text gspace textNaOH times frac1 text mol40text g 225 space textmol NaOH. The equivalent mass of a salt is defined as the mass of the salt formed when one equivalent of an. HCl does not have a high molecular mass.
What are the molecular weights of the following compounds. What is the difference between molarity and molar mass. Convert between NaOH weight and moles.
Magnesium Chloride Structure MgCl 2. It is used to express concentration of a particular solution. This gives water a molecular mass of 18Acetic acid.
A unit of concentration equal to the number moles of solute in a 1L of solution. CO3- because the background. If you want to find the molar mass you need to know how many grams of acid are in 1 mole or if you knew how many moles were in the sample above then you could calculate the molecular mass.
This compound is also known as Sodium Hydroxide. Mass percent composition Atomic percent. How do you answer this question.
Magnesium Chloride Structure MgCl2. Sodium bicarbonate baking soda NaHCO3 can be purified by dissolving it in hot water 60 C filtering toexample. Of moles Given mass of compound in the questionMolecular mass of the given compound Thanks.
Finding the equivalent mass of salts is quite interesting. If the equation is arranged correctly the mass units g cancel out and leave moles as the unit. Molar mass of NaOH is 3999711 000037 gmol Compound name is sodium hydroxide.
Melting Point of Magnesium Chloride. Examples of molecular weight computations. Not Helpful 16 Helpful 109.
Elemental composition of NaOH. Convert grams NaOH to moles or moles NaOH to grams. Do a quick conversion.
The molar mass of KHPh is 20423 gmole and it has one acidic proton which will react quantitatively with OH. Amedeo Avogadro is considered to be the number of units usually molecules or atoms in a substance that is proportional. The molecular weight of sodium hydroxide is 40 gmol.
A hot water solution containing 731 mass of NaOH is an eutectic that solidifies at about 6263 C as an intimate mix of anhydrous and monohydrate crystals. 95211 gmol anhydrous Density of Magnesium Chloride. Compound Moles Weight g.
2 48gmole 602x10. NaOH molecular weight. Substance MW or FW molar mass Fe 55.
The molar mass of NaOH sodium hydroxide is. Check the chart for more details. 1 mole 602 x 1023 particles 1 mole molar mass could be atomic mass from periodic table or molecular mass.
Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Now molecular mass calculator add all masses of substances together. 2298977 159994 100794 Percent composition by element.
The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol. Molarity is a measure and unit of concentration. 1M solution of NaCl 23Na 35Cl 58 g.
In this example you have 250 ml of solution. Equivalent Mass of Salts. 16 g mol 23 g mol 1 g mol 16 g mol 40 g mol 23 g mol 1 g mol 40 g mole.
If you need to prepare roughly one liter of 1 M NaOH solution you dissolve the molar mass of NaOH 400 g using distilled water in a beaker then transfer this solution to a one liter volumetric. It solidifies at. On the other hand molar mass is a unit of mass.
232 gcm 3 anhydrous Boiling Point of Magnesium Chloride. So how many moles of acid are. Element Symbol Atomic weight Atoms Mass percent.
The molecular mass of NaOH is 40 so work out 1 40 0025. Since we are asked to find the mass of Na_2SO_4 formed in this reaction we need to multiply the answer of step 3 to the ratio of the formula mass of Na_2SO_4. Molar Mass of sodium hydroxide NaOH Solving for the atomic mass of sodium hydroxide NaOH Need to know the atomic mass of a sodium hydroxide molecule.
See also our theoretical yield calculator for chemical reactions probably your next stop to finish the problem set. Has a medium to high molecular weight For this experiment a solution of NaOH which has an approximate concentration of 01 M will be standardized using potassium acid phthalate KHPh. Definitions of molecular mass molecular weight molar mass and molar weight.
Molar mass of NaOH 3999711 gmol. OHaq KHPh aq H2O l KPhaq For the highest accuracy a sample size is. This the same as multiplying by the reciprocal of 40 gmol.
The result from the above calculation will then be multiplied by the mole ratio of Na_2SO_4 and NaOH which is 1 mol Na_2SO_42 mol NaOH. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. The third stable eutectic has 184 mass of NaOH.
Its more like the reaction between the acids and bases. The hydrogens atomic mass and number are both 1 while oxygens number is 8 and its mass is 16. Considering that the molecular weight of KHP is MWKHP 20423 gmol the concentration of the KHP solution is.
Trial 1 Trial 2 Trial 3 Initial volume mL 150 050 2460 Final volume. Work out 0025 025 01. As you will soon see in the technique section of this laboratory this experiment requires that you weigh a certain mass of the unknown salt dissolve it into a solvent and pass it over a column of ion-exchange resin.
Next divide the number of moles of solute by the number of liters of solution. MgCl 2 Uses Magnesium Chloride Magnesium Chloride is used as a precursor magnesium metals. As such it is defined and can be calculated as follows.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. 1 moles NaOH 3999711 gram using the molecular weight calculator and the molar mass of NaOH. All masses must be to nearest hundredth 1 NaOH 2 H 3 PO 4 3 H 2 O 4 Mn 2 Se 7 5 MgCl 2 6 NH 4 2 SO 4 There are three definitions equalities of mole.
H 3 x 1. How do you make a 20 NaOH solution. Visit BYJUS for more information.
48149 g 0250 L 00943035 M 943035 mM KHP 20423 gmol c The titration standardization results using 2500 mL aliquots of the KHP solution are summarized in Table 1 below.