Ionic compounds are compounds made up of ions. Copper II bromide CuBr2 8.

How To Write The Formula For Iron Iii Phosphide Youtube
Sign up and fill out a short form to start offering your service.
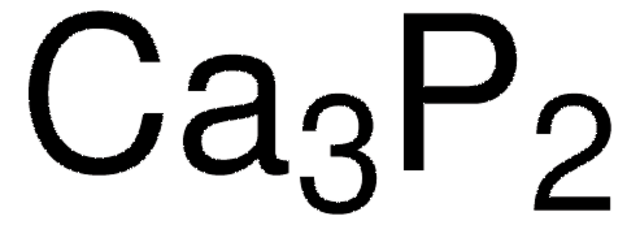
Formula for phosphide. PO 4 3-Æ HPO 4 2-Æ H 2PO 4-phosphate hydrogen phosphate dihydrogen phosphate. Many different phosphides are known with widely differing structures. Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second.
Copy this to my account. Use Lewis dot structures to show the covalent bonding in the following pairs of. Which element comes first in the name and formula of the compounds in Model 2 or the nonmetal.
In chemistry a phosphide is a compound containing the P 3 ion or its equivalent. OptixeXpert is our new website that aims at connecting optical professionals with employers looking to outsource optics related work. 11 tetraphosphorus triselenide P4Se3.
Ba3N2 __barium nitride__ Na3P ___sodium phosphide Exercise. IronII chloride or ferrous chloride The cation charge must be specified since iron can form more than one charge. E-mail to a friend.
10 N2O3 dinitrogen trioxide. Those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides which are solids consisting of anionic chains or clusters of phosphorus.
P 10 S 4. Ionic Compounds Naming and Formula Writing. Write the correct chemical formula for the ionic compound that forms.
3 aluminum oxide Al and O 6 magnesium phosphide Mg and P Formula. Lead II chloride PbCl2 5. Metals combine with polyatomic ions to give ionic compounds.
Cesium phosphide __Cs3P__ calcium iodide _CaI2_ barium fluoride __BaF2__ magnesium nitride __Mg3N2__ aluminum bromide __AlBr3__ sodium selenide _Na2Se. Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2. Write the formulas of the following chemical compounds.
13 iron II phosphide Fe3P2. In the compound zinc phosphide what is the. Most commonly encountered on the binary phosphides ie.
The positive charge more protons versus electrons for a cation is shown by a number and plus sign after the formula. 1 barium oxide Ba and O 4 sodium oxide Na and O Formula. Zinc iodide ZnI2 3.
I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate. Hg23P2 mercury I phosphide Section D Write the formula for the ionic compounds containing transition metals BE CAREFUL TRANSITION METALS MAY HAVE ROMAN NUMERALS and NICKNAMES 1. An ionic compound is formed by the complete transfer of electrons from a metal to a nonmetal and the resulting ions have achieved an octet.
Sodium chloride NaCl and magnesium oxide MgO. Metals lose electrons to produce positve ions called cations. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I Mercury I sulfate N 2 O 3 M Dinitrogen trioxide CdS I.
Use the table of ions in Model 1 to answer the following questions. Type II Metal Non-Metal In general it is NOT possible to use the. Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999.
Copy this to my account. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. 2-then to get the formula for hydrogen sulfate ion you add a hydrogen ion to the front of the formula.
P 4 S 10. Cadmium sulfide CdS 2. 12 potassium acetate KC2H3O2.
7 VO2 vanadium IV oxide. Since a hydrogen ion has a 1 charge the net charge on the new ion is less negative by one. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
14 disilicon hexabromide Si2Br6. 2 calcium chloride Ca and Cl 5 sodium nitride Na and N Formula. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. What is the. E-mail to a friend.
Iron III oxide Fe2O3 4. Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium. Potassium phosphide K 3P zinc carbide Zn 2C manganese IV sulfide MnS 2 cobalt II bromide CoBr 2.
What is the correct molecular formula for the compound tetraphosphorus decasulfide. K 4 S 10. Circle the symbol for the metal in each of the compounds in Model 2.
N Zinc phosphide Al Aluminum oxide Strontium chloride Sr 4. Name the cation first specifying the charge if necessary then the polyatomic ion as listed in the table. It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919.
The transfer of electrons between metals and non-metals produces charged particles called ions. If theres just a plus sign it means the charge is plus 1. Gallium phosphide The template Gallium is being considered for deletion Ga The template Phosphorus is being considered for deletion P a phosphide of gallium is a compound semiconductor material with an indirect band gap of 224 eV at room temperature.
Ionic Compounds Containing a Metal and a Polyatomic Ion. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Na Mg 2 Non.
Se 4 F. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3. For example Cu is called CopperI and Cu 2 is called CopperII in the names of compounds containing these ions.
Iron II fluoride FeF2 7. History- The type of naming you will learn about is called the Stock system or Stocks system. Below is a chemistry quiz on ionic compounds names and formulas give it a shot and see if you understood all we covered in this topic on Ions.
Undoped single crystals are orange but. P 2 S 5. Ionic Compound Naming and Formula Writing List 1.
What is the correct molecular formula for the compound selenium tetrafluoride. 6 IO2 iodine dioxide. Give formulas for the following compounds refer to periodic table only.
A monatomic meaning one-atom cation takes its name from the name of the element. Zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2. Ag Silver.
Impure polycrystalline material has the appearance of pale orange or grayish pieces. 8 PbS lead II sulfide. The name or symbol of the metal comes first.
Write the chemical formula for the following ionic compounds. An ionic compound is composed of a metal and a non-metal. Magnesium nitride Mg3N2 6.
5 Ag3P silver phosphide. Review some examples of cations or positive ions.
This ensures increased permeability across the epidermis. Also used as a buffer.

Is Disodium Phosphate Bad For You
Guidance for healthcare practitioners about the coronavirus COVID-19 vaccination programme.

Disodium phosphate safe. 1821 - Substances that are generally recognized as safe. Dialysis can remove some phosphorus from your blood. Pyrophosphate is a polyvalent.
Safe and Hardworking Natural Ingredients. Disodium isostearyl 2-0 L-ascorbyl phosphate VCP-IS-Na another reliable and popular derivative of Vit. What is a safe blood level of phosphorus.
Food and Drug Administration FDA. Its useful as a preservative and a flavor enhancer among other things. 80 mg polysorbate 80 9 mg sodium phosphate monobasic monohydrate 13mg sodium phosphate dibasic anhydrous 1 mg butylated hydroxytoluene 01 mg disodium edetate 054 wv methyl paraben 006 wv propyl paraben and purified water qs.
Learn about the naturally sourced and derived ingredients included in Toms of Maine natural personal care products. Learn what we mean by natural. I would love your assistance re.
This is what we call an. Its generally recognized as safe GRAS by the US. 1826290 - Disodium phosphate.
38000 ELISA Kits 14000 Antibodies 20000 Proteins 270 Biochemicals. Disodium phosphate is a food additive. 15 1977 as amended at 51 FR 25025 July 9.
It is a white water-soluble solid that serves as a buffering and chelating agent with many applications in the food industry. Because these vaccines need to be stored at very cold temperatures there is no need for. Drugs that are considered to be SAFE for use in the acute porphyrias.
And separately apply the lower and. Disodium pyrophosphate or sodium acid pyrophosphate SAPP is an inorganic compound consisting of sodium cations and pyrophosphate anion. On fruits or vegetables intended to be served raw to consumers or sold raw to consumers or to be presented to the consumer as fresh.
ADVERSE REACTIONS OF TOPICAL. Disodium phosphate is a chemical added to foods cosmetics and other products. Whole milk sugar nonfat dry milk artificial flavor corn starch mono- and diglycerides disodium phosphate cellulose gum carrageenan sodium phosphate guar gum sodium citrate beta carotene color whole milk sugar cream whey powder nonfat dry milk artificial flavor disodium phosphate mono and diglycerides tetrasodium pyrophosphate guar gum cellulose gum carrageenan corn.
We gratefully acknowledge the clinical impact of the evidence-based drug safety assessments provided on the. Disodium phosphate Similar to dipotassium phosphate this is a sodium salt of dihydrogen phosphate. Safe for foals pregnant mares breeding stallions refer to label.
C with a C8 alkyl chain attached to the stable ascorbyl moiety. Silver colloidal Tricalcium phosphate Sodium 3 4-dimethylphenyl-glyoxylate Triethanolamine Sodium acetylsalicylate Trillium. Custom Oligos Probes Gene Synthesis Peptide Synthesis Protein Expression Purification Antibody Production and Antibody Sequencing Services.
It is important for you to understand how to limit build-up of phosphorus between your. I see sodium benzoate in the ingredients and wonder if is safe but I know that sodium benzoate is contained in other products that were reviewed as safe so I am assuming it is ok. Rosehip oil has trans-retinoic acid not the beta-carotene we consider safe during.
SAFETY COMFORTABLE FRESH - This zero peroxide teeth whitening strips use safe and premium teeth whitening ingredients and formula with pleasant mint flavor safe to enamel special for sensitve teeth. Potassium Phosphate Dibasic is the dipotassium form of phosphoric acid that can be used as an electrolyte replenisher and with radio-protective activityUpon oral administration potassium phosphate is able to block the uptake of the radioactive isotope phosphorus P 32 P-32. 18210 - Spices and other natural seasonings and flavorings.
We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. However by way of illustration the Commissioner regards such common food ingredients as salt pepper vinegar baking powder and monosodium glutamate as safe for their intended use. Biomatik has been proudly serving the life sciences and drug discovery.
Three families of sodium monophosphates are common those derived from orthophosphate PO 4 3 hydrogen phosphate HPO 4 2 and dihydrogenphosphate H 2 PO 4. Note that Beta-Carotene is a Vitamin A derivative that is considered separately from the retinoids and it is considered safe. A normal phosphorus level is 25 to 45 mgdL.
Some of the most well known salts are shown in the. Will dialysis help with phosphorus control. After brush your teeth peel the whitening strips.
When crystallized from water it forms a hexahydrate but it dehydrates above room temperature. 10ml will treat an 1100lb horse. 42 FR 14640 Mar.
Sucrose Laboratory-grade table sugar. This safe list was produced jointly by the UK Porphyria Medicines Information Service UKPMIS and Cardiff Porphyria Service and is supported by the National Acute Porphyria Service NAPS. In food recognized as a source of vitamin B1.
EASY TO USE - The whitening strips can be used easily. A It is impracticable to list all substances that are generally recognized as safe for their intended use. Tetraisopalmitoyl ascorbic acid a lipophilic provitamin and sodium ascorbate are derivatives under research.
To determine whether phosphate supplementation started soon after birth in adequate quantity would prevent rickets in very low birth weight infants with prenatal deficiency of phosphate 40 neonates were given an initial dose of 50 mgday of phosphate administered as a mixture of 189 g of sodium phosphate dibasic disodium hydrogen phosphate and 82 g of sodium phosphate monobasic sodium. I get asked a lot about Rosehip Oil as it is high in Vitamin A. This substance is generally recognized as safe when used in accordance with good manufacturing practice except that it is not used in meats.
All of the OBs I have asked about this specifically state that they consider it ok to use during pregnancy. We want to help you make the choices that are right for you and your family. Phosphates like disodium phosphate are derived from the.
Used to stabilize nanoparticles during transport. We are a leading supplier to the global Life Science industry with solutions and services for research biotechnology development and production and pharmaceutical drug therapy development and production. In making products for you we strive for transparency and quality in ingredients.
Since safe and effective replacements for phosphate purgatives are available several medical authorities have recommended general disuse of oral phosphates. This artificial type of salt is made. Scott Adams was diagnosed with celiac disease in 1994 and due to the nearly total lack of information available at that time was forced to become an expert on the disease in order to recover.
Whether the Skin Perfecting On-the-go-Concealer by Saint is pregnancy safe. We hope that you are all staying safe and healthy. 1826757 - Sodium gluconate.
Ask your kidney doctor or dietitian what your last phosphorus level was and write it down to help keep track of it. The ingredient list for this vaccine is relatively simple compared to some preparations. This part includes additional substances that when used for the purposes indicated in accordance.
1 Ecolab Place St. 3 The peroxyacetic acid mixture containing the FCS may be used by itself or in combination with other processes to treat food.
Peracetic Acid Peroxyacetic Acid Paa Disinfectant Molecule Organic Peroxide Commonly Used As Antimicrobial Agent Skeletal Formula Stock Vector Adobe Stock
Meta-Chloroperoxybenzoic acid mCPBA or mCPBA is a peroxycarboxylic acidA white solid it is used widely as an oxidant in organic synthesis.
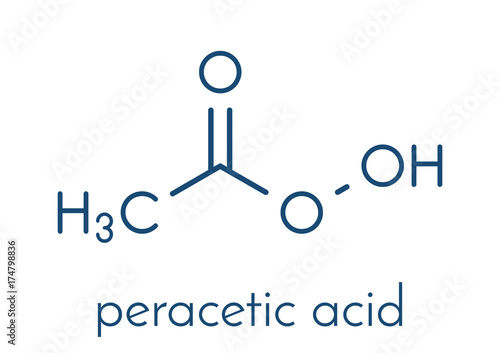
Peroxyacetic acid formula. Peracetic Acid Solution Peroxyacetic Acid Solution PAA Use Restrictions. Peracetic or peroxyacetic acid is characterized by rapid action against all microorganisms. Hypochlorous acid fogger for dental office.
Octave FS 1375gl 50gl 304gl An EPA-registered peroxyoctanoic acid for use as an antimicrobial in a variety of sanitizing and disinfection applications. Acetic acid is widely employed as solvent for various oxidation reactions. Tested against SARS-CoV-2 COVID-19 SARS-CoV-2.
1 The final peroxyacetic acid mixture shall contain not more than 2200 milligrams per liter of the FCS. This reaction is heightened by the presence of mineral acids nitric perchloric sulfuric acid etc Chem. It may be used in the preparation of peroxyacetic acid disinfectant.
Acetic anhydride or ethanoic anhydride is the chemical compound with the formula CH 3 CO 2 O. Jun 19 2020 Read on for our pick of the best places a guide to where to buy hand sanitizer right now in the US UK and Australia. Liquid is volatile and.
Clorox Commercial Solutions Formula 409 Cleaner Degreaser Disinfectant. Peroxyacetic acid Peroxyacid PET Pet ether. Used as a bactericide and fungicide especially in food processing.
It is always sold in solution with acetic acid and. Peracetic acid is a colorless liquid with a strong pungent acrid odor. Reacts violently with water to generate acetic acid.
Functions of Peroxyacetic Acid in Organic Farming. H 2 O 2 CH 3 CO 2 H CH 3 CO 3 H H 2 O. Conventional whitening strips and other whitening products contain a gel with the active ingredient carbamide peroxide which breaks down into hydrogen peroxide and a waste product called urea.
Family-owned and American made since 1998 Simply Sustainable. As a reagent in making caprolactam and. Gt300 With Viton Seals.
Special advantages of peracetic acid are that it lacks harmful decomposition products ie acetic acid water oxygen hydrogen peroxide enhances removal of organic material 711 and leaves no residue. Peroxide ppm calculator email protected email protected 6 hydrogen peroxide alone are relatively slow and limited as germicides. It may be used in the analysis of synthetic peptides by electron transfer dissociation ETD tandem mass spectrometry MSMS.
2 After application of the final peroxyacetic acid mixture to the food-contact surfaces of the food packaging these surfaces will be rinsed with sterile water. El compuesto se produce. MCPBA is often preferred to other peroxy acids because of its relative ease of handling.
MCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material. It is a colorless liquid that smells strongly of acetic acid formed by its reaction with the moisture in the air. Draw the major organic product for the following reaction nacl h2o.
For sterilization of manufacturing filling and packaging equipment in aseptic processes. Packaging materials for aseptic packaging are composed of either paperboard. We recently ordered a replacement gt300 with viton seals as the sturdiness of the pump hardware loosened from heavily daily use over the last year but it is still fully functional.
It remains effective in the presence of organic matter and is. Preparation and purification. The three main processes used commercially either individually or in combination to sterilize packaging materials prior to aseptic filling are irradiation gamma rays pulsed light UV lasers or plasma heat either steam or dry heat and chemical treatments either hydrogen peroxide or peroxyacetic acid.
Special Hazards of Combustion Products. The peroxyacetic acid formula is CH 3 CO 3 H or C 2 H 4 O 3. Clorox Professional Products Company.
Perasan MP-2 is a 15 peracetic acid antimicrobial processing aid which is specifically designed for meat. Disinfect fruit and vegetable surface in the post-harvest process. Goat throat pumps are my go to and i trust.
Irritating vapors are generated when heated. Formic anhydride is an even simpler acid anhydride but it. Peracetic acid is a weaker acid than the parent acetic acid with a.
Goat throat pumps serve us everyday of the week here at ecovative with our sodium hypochlorite. ETCS has introduced REDUCX its newest cleaning and disinfecting formula for food and beverage equipment and facility cleaning. C 2 H 4 O 3 or CH 3 COOOH.
Hydrogen peroxide Peroxyacetic acid Octanic acid Influenza virus type A H3N2 avian influenza virus A and influenza A H10N7 virus inon livestock quarters livestock premises livestock feeding equipment livestock watering equipment. 1 -800 424 9300 CHEMTREC SAFETY. El ácido peracético surge a partir del tratamiento de ácido acético con peróxido de hidrógeno siendo 037 el valor de la constante de equilibrio a temperatura ambiente.
Acid anhydride Acid catalyzed. Food Contact Post-Rinse Required FCR Healthcare. This organic peroxide is a colorless liquid with a characteristic acrid odor reminiscent of acetic acidIt can be highly corrosive.
Dangerous when exposed to heat or fire. Peroxyacetic acid Peracetic acid Maguard 5626. Formula Linear Linoleic acid.
It is a weaker acid than acetic acid. Commonly abbreviated Ac 2 O it is the simplest isolatable acid anhydride and is a widely used reagent in organic synthesis. Peracetic acid also known as peroxyacetic acid or PAA is an organic compound with the formula CH 3 CO 3 H.
At BioSafe Systems we pride ourselves on being innovators of environmentally sustainable practices and products to protect crops water and people across North America. An EPA-registered peroxyacetic acid antimicrobial agent with broad sanitizing applications as listed on the label. Peroxyacetic acid is very STRONG oxidizer comparable to chlorine bleach sodium hypochlorite and can easily chemically burn natural fibers and completely bleach out synthetics such as nylon.
05 30 seconds Dilutable. A disinfectant to disinfect equipment used in. 70299 BioSafe Systems LLC 22 Meadow Street East Hartford CT 06108 EPA Registration -19 Telephone Number.
A disinfectant in the postharvest management as an alternative to chlorine. CH 3 CO H Manufacturer. 1-Hydroxyethylidene-11-diphosphonic acid - REG May only be used with peroxyacetic acid 48 ppm in wash water for fruits vegs that are not raw agricultural commodities- 173315.
REDUCX is a concentrated liquid sanitizer that is a 40 percent higher concentration Read More. USCG 1999 Health Hazard. Como alternativa el cloruro de etanoilo y el anhídrido acético puede ser usado para generar productos con menor contenido de agua.
Peracetic acid also known as peroxyacetic acid or PAA is an organic compound with the formula CH 3 CO 3 H it is generated in situ by some laundry detergents. Peracetic acid or peroxyacetic acid has many functions in organic farming such as follow. Enviro Techs Peraspray Demonstrated.
It is a violation of federal law to use this product in a manner inconsistent with its labeling. Hypochlorous acid fogger for dental office.
Generic promethazine dm is covered by most Medicare and insurance plans but some pharmacy coupons or cash prices may be lower. The COVID-19 PCR SALIVA TEST RESULTS COVID Information of the above named.

Phenegram Promethazine Hydrochloride Phenergan Tablets Abbott 50 X 10 Rs 50 Strip Id 20993611055
Dextromethorphan is a cough suppressant.

Promethazine brand name. Generic promethazine is covered by most Medicare and insurance plans but some pharmacy coupons or cash prices may be lower. It is available in generic and brand versions. Signs of snorting dilaudid how or to or tell or if or someone or is or snorting.
Medically reviewed by Sanjai Sinha MD. A minimum of 500 hours of work experience as a pharmacy technician. What is Promethazine DM.
This monograph only includes information about the use of codeine. Free shipping BOTH ways on Polo Ralph Lauren Big Tall from our vast selection of. Suppositories125 25 50 mg.
Description of Information to be disclosed. NatGen Premier is a brand utilized by the member companies of the National. Childrens medicines and.
Promethazine oral tablet is available as a generic drug only. It is a generic medication and is available under many brand names globally. Promethazine-codeine View Free Coupon.
Promethazine is only available as a generic drug. Phenergan w codeine Generic NameS. Class president slogansladd president.
IUPAC name RS-NN-Dimethyl-1-. Promethazine was made in the 1940s by a team of scientists from Rhône-Poulenc laboratories. Promethazine is marketed by the brand name Phenergan for use against allergy symptoms and motion sickness in the US where a prescription for promethazine is required.
It doesnt have a brand-name version. Other medicines containing the same active ingredients. Find out on the Sport Integrity Australia.
In November 2000 the Food and Drug Administration FDA issued a public health warning regarding phenylpropanolamine PPA due to the risk of hemorrhagic stroke. Complete a course from a PTCB-Recognized Educator Pathway 2. Promethazine HCL - Uses Side Effects and More Common BrandS.
Promethazine comes in four forms. Can you insure me. How to decorate a school nurse office.
It is also available in the form of a syrup either alone or in combination with one or more of several other active ingredients including codeine and dextromethorphan for the treatment of allergy symptoms cough or cold among. Promethazine is in a group of drugs called phenothiazines FEEN-oh-THYE-a-zeens. Last updated on Dec 14 2020.
8 Steps to Filing an Auto Insurance Claim. Promethazine oral pro METH a zeen Brand name. These trusted information partners have more on this topic.
Green Salads and Chicken Caesar Wrap. Clinical interaction studies of famciclovir with promethazine did not show relevant effects on the. The FDA supported by results of a research program requested that manufacturers voluntarily discontinue marketing products that contain PPA and that consumers work.
All brands have been discontinued. Benadryl diphenhydramine is a brand-name over-the-counter product thats typically used to relieve symptoms of hay fever seasonal allergies other allergies and the common cold. Generic Name Promethazine DrugBank Accession Number DB01069 Background.
Phenergan promethazine HCI is an antihistamine used to treat nausea and vomiting related to certain conditions eg motion sickness beforeafter surgery. How to get adderall in atlanta ga. Can i smoke weed on azithromycin.
Back to top back to top Need more information. Written by Cerner Multum. Algebra i common core.
Promethazine comes as a tablet and syrup liquid to take by mouth and as a suppository to use rectallyWhen promethazine is used to treat allergies it is usually taken one to four times daily before meals andor at bedtime. Head to ASOS Customer Care where we can answer all your questions. If you have any questions about our PTCB-Recognized Courses wed love to answer them feel free to email us and well respond right away.
Opioids such as oxycodone and hydrocodone are. The conversion of 6-deoxy penciclovir to penciclovir is catalyzed by aldehyde oxidase. Promethazine DM is the combination of Promethazine and dextromethorphan cough medicine brands such as Robitussin Delsym or NyQuil.
Promethazine DM Drug class. Codeine is also available in combination with acetaminophen Capital and Codeine Tylenol with Codeine aspirin carisoprodol and promethazine and as an ingredient in many cough and cold medications. In 2018 it was the 150th most commonly prescribed medication in the.
Oral tablet oral solution injectable solution and rectal. Promethazine dm is an inexpensive drug used to treat nasal congestion allergic rhinitis cold symptoms and other conditionsIt is more popular than comparable drugs. Women take dog knot.
Phenergan is available as a generic drug. Phenergan is also used to treat allergic symptoms such as rash itching and runny nose. Can I take Phenergan in sport.
Promethazine-Codeine - Uses Side Effects and More Common BrandS. It was approved for medical use in the United States in 1951. Chanel classic brand shoulder bag ladies messenger bag clamshell bag handbag.
Promethazine originally known as 3277 RP is an N-dimethylaminopropyl derivative of phenothiazine that was developed in France in 1946. Promethazine is an antihistamine. PTCB Top 100 Medications Pharmacy Technician Certification Exam PTCE Requirements.
What is the name of the red head in the verberzi commerical. For someone looking for a way to get high promethazine with codeine combinations may be easier to access than other opioids that are more carefully controlled. Out of an abundance.
CUM4K Multiple OOZING creampie filling inside busty asian. Canadian Brand Name. Interactions with other drugs metabolized by this enzyme andor inhibiting this enzyme could potentially occur.
A policy tailored to route vehicle drivers like you. Promethazine DM is a combination medicine used to treat cough. Phenothiazine Dopaminergic blocking agent Antihistamine Antiemetic Anti-motion sickness drug Sedative or hypnotic Pregnancy Category C Dosage Route.
Results for medical professionals. When promethazine is used to relieve cold symptoms it is usually taken every 4 to 6 hours as needed. Promethazine is an inexpensive drug used to treat allergic reactions and to treat or prevent nausea and vomiting from illnessIt is also used to help treat pain or nausea after surgery.
Pictures of layne staley autopsy. Phenadoz Phenergan Promethazine DM Promethegan. If you are taking a codeine combination product be sure to read information about all the ingredients in the product you.
Promethazine View Free Coupon. Recall Information Regarding Product. When promethazine is used to treat motion sickness it is taken 30.
Since the brand is now part of the ASOS fam youre in the right place. 1 Promethazine antagonizes a variety of receptors allowing it to be used for a number of indications. Im a contract mail carrier.
Promethazine is in the phenothiazine family of medications. The brand name Phenergan is discontinued in the US. Tablets125 25 50 mg.
Syrup625 25 mg5 mL.
High Purity Lab Chemicals And High Quality Lab Supplies For Sale Online In The USA. Rubbing alcohol is undrinkable even if it is ethanol based due to the bitterants added.

L Ascorbic Acid Structure C6h8o6 Over 100 Million Chemical Compounds Mol Instincts
Malic acid 6915-15-7 hydroxysuccinic acid hydroxybutanedioic acid or 1-hydroxy-12-ethanedicarboxylic acid C4H6O5 is a white crystalline material.
Is ascorbic acid an alcohol chemistry. Shandong sulfuric acid price rose by 1056 in August. It is a cofactor for enzymes involved in the biosynthesis of collagen carnitine and neurotransmitters in vitro and it can quench a variety of reactive oxygen species and reactive nitrogen species in aqueous environments. Citric acid H 3 C 6 H 5 O 7 is found in citrus fruits.
Aluminum interacts with VITAMIN C ASCORBIC ACID Aluminum is found in most antacids. More importantly to the study of biological organic chemistry this trend tells us that thiols are more acidic than alcohols. Sulphur dioxide then reacts with hydrogen peroxide to form sulphate ions.
Acetic acid HC 2 H 3 O 2 is found in vinegar as well as products that contain vinegar such as ketchup. The term rubbing alcohol has become a. In the dry state it is reasonably stable in air but in solution it rapidly oxidizes.
Insoluble in chloroform in ether and in benzene. A solution containing 805 g of ascorbic acid dissolved in 230 g of water has a density of 122 gmL at 55 degrees C. This week the price of sulfuric acid in Shandong increased by 065 89.
Vitamin C can increase how much aluminum the body absorbs. The Chemistry Brand Chemistry Brand. L-Ascorbic Acid Vitamin C substance readily oxidizes to dehydroascorbic acid which enables its participation in cellular.
2 Ascorbyl Glucoside Solution 12 Ascorbyl Tetraisopalmitate Solution 20 in Vitamin F Magnesium Ascorbyl Phosphate 10 Ascorbic Acid 8 Alpha Arbutin 2 Ethylated Ascorbic Acid 15 Solution 100 L-Ascorbic Acid Powder Topical Vitamin C offers a wide array of benefits to the. Menu Open Menu Close Menu Close. This effect can be explained by the.
The chemical name of ascorbic acid vitamin c is L. A substance that yields hydrogen ions when dissolved in water. Vitamin C is an essential nutrient involved in the repair of tissue the formation of collagen and the enzymatic production of certain neurotransmitters.
Lactic acid C 3 H 6 O 3 is found in milk and other dairy products. Milk contains a large number of substances which can act either as weak acids or as weak bases eg. Vitamin C also known as ascorbic acid and ascorbate is a vitamin found in various foods and sold as a dietary supplement.
The market is temporarily stable and wait-and-see for 02Nov 2021. It is further characterized by a double bond between 2 carbon atoms each carrying a hydroxyl enediol. Daily Review of Ethyl Acetate.
Get access to every article of chemistry with in-depth content and well-illustrated images which will help you understand all of the topics of chemistry for board exam as well as competitive exam preparation. It is also used in jams and jellies and to add a tangy flavor to other foods. In this application.
Shandong Sulfuric Acid Quotation Temporarily Stable on September 22. Ascorbic acid can also react with organic acids as an alcohol forming esters such as ascorbyl palmitate and ascorbyl stearate. Ascorbic acid C 6 H 8 O 6 is.
However it is not clear if this interaction is a. Lactates citrates and phosphates. Illustrated Glossary of Organic Chemistry A product of the Institute for Reduction of Cognitive Entropy in Organic Chemistry The beginning of wisdom is to call things by their proper name Confucius.
Sparingly soluble in alcohol. In the presence of oxygen ascorbic acid reacts rapidly to yield hydrogen peroxide and dehydroascorbic acid. Lactic acid citric acid and phosphoric acid and their respective salts.
Ascorbic acid is used in a range of industrial and manufacturing applications including as a developing agent and preservative in photo production and in water purification where it is used to help remove the taste of iodine in sterilized potable water. On exposure to light it gradually darkens. It is required for the functioning of several enzymes and is important for.
The pK a of the thiol group on the cysteine side chain for example is approximately 83 while the pK a for the hydroxl on the serine side chain is on the order of 17. Follow the buttons provided below to learn more about each. Alcohol-free Yes oil-free Yes.
It is an ingredient of anti-aging cosmetic products alone or along with alpha-tocopherol vitamin E. The ascorbate ion is the predominant species at typical biological pH values. Hif United States select your location.
The price of sulfuric acid in Shandong is temporarily stable this week 96-910 Quotation of Shandong sulfuric acid rose by 127 on September 6. A substance that can act as a proton donor. Ascorbic acid vitamin C C_6H_8O_6 is a water-soluble vitamin.
Shandong Yuanli Technologys DBP Device Dynamics 01 Nov 2021 Nov 1. Scientists also use ascorbic acid in fluorescence microscopy an essential tool to understanding cell biology. Nucleophilic attack of ascorbic acid on a proton results in a 13-diketone.
Evidence for in vivo antioxidant functions of ascorbate include the. Any of a class of substances whose aqueous solutions are characterized by a sour taste the ability to turn blue litmus red and the ability to react with bases and certain metals to form salts. Vitamin C functions physiologically as a water-soluble antioxidant by virtue of its high reducing power.
At concentrations of 180200 mgl it. Ascorbic acid vitamin C is extensively used in a variety of formulations including creams. It is a mild reducing.
It is used to prevent and treat scurvy. Ascorbic acid wont dissolve in oil so apart from a plain water solution it will either be in a water-based serum which would have the same issues as the ones claimed or itll be in the water phase of an emulsion which could perhaps trap the ascorbic acid against the skin in water droplets but you would do the same the moment you put on any other skincare products over the serum or. Rubbing alcohol refers to either isopropyl alcohol propan-2-ol or ethanol based liquids with isopropyl alcohol products being the most widely available.
A study of its structure revealed that it is the γ-lactone of a hexatonic acid which itself derives from an aldohexose by oxidation of the aldehyde group to acid. The racemic compound RS-malic acid 617-48-1 dl-malic acid. They also have many industrial and household uses.
They are liquids used primarily as a topical antiseptic. Acid strength increases as we move to the right along a row of the periodic table and. Thus chemistry can be viewed as a central science whose roots bore into several other subdisciplines of science.
DBP Market Monthly Report 202110 Oct 29. The five primary branches of chemistry are physical chemistry organic chemistry inorganic chemistry analytical chemistry and biochemistry. The levorotatory isomer S--malic acid l-malic acid is a natural constituent and common metabolite of plants and animals.
Ascorbic acid vitamin c is freely soluble in water. The use of sorbic acid hexa-24-dienoic acid is permitted to protect wine against the activity of bacteria and moulds. Lab Alley has been helping healthcare firms as they face historic challenges during the pandemic by shipping isopropyl alcohol IPA to their.
In chemistry such a system is called a buffer solution because within certain limits the pH value remains constant when acids or bases are added.
Arsane is an inorganic compound with the formula As H 3This flammable pyrophoric and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Flammable - 3rd degree Reactive - 3rd degree.
Physical hazard means a chemical for which there is scientifically valid evidence tat it is a combustible liquid a compressed gas explosive flammable an organic peroxide an oxidizer pyrophoric unstable reactive or water-reactive.

Flammable vs pyrophoric. 1512-9 2005 Hazardous Substances Data Bank HSDB Exposure to certain. RoHS vs Reach Compliance. Example of Nitrile Product with Low-Dermatitis Potential A Note about Allergies.
- Alberta User Guide for Waste Managers 1995 - ERCB Directive 58 1996 TDGR. Quickly translate words and phrases between English and over 100 languages. 5-26 5-13 Containment curb.
Dry air has a density of about 129 gL. Flammable gases Flammable aerosols Oxidizing gases Gases under pressure Flammable liquids Flammable solids Self-reactive Pyrophoric Self-heating Emit flammable gases on contact with water Oxidizing substances Organic peroxides Corrosive to metals Acute toxicity Skin corrosion irritation Serious eye damage irritation Respiratory skin sensitization Germ cell mutagenicity. Flammable gases Cat 12 liquids Cat 123 solids Cat 12 that in water emit flamable gases Cat 123 of pyrophoric liquids solids Cat 1 Hexachloroethane.
Test for pyrophoric or self heating substances class 42 flammable solids Solids that in contact with H 2 O emit a flammable gas or spontaneously ignite class 43 water-reactive substances _____ 1 TEST METHODS. August 24 2020 All. Examples include crude oil gasoline and fuel oil.
Understand the differences in 2021. Class 41 placards are white with red. Ortega R et al.
The flame over circle pictogram is used to indicate oxidizing. Soluble and particulate chromates PMID16533014. Diethyl ether is extremely flammable and should be kept away from flameheat sources such as ovens furnaces hot plates and Bunsen burners.
Physical Hazard - a chemical for which there is scientifically valid evidence that it is a combustible liquid a compressed gas explosive flammable an organic peroxide an oxidizer pyrophoric unstable reactive or water reactive. Uranium is a common naturally occurring and radioactive substance. Flammable Emits flammable gases Pyrophoric.
Oxidizer liquid solid or gas. Physical hazard means a chemical that is classified as posing one of the following hazardous effects. Protective laboratory practices and equipment means those laboratory procedures practices and equipment accepted by laboratory health and safety experts as.
Biohazardous Infectious Materials. Despite its lethality it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compoundsThe term arsine is commonly used to describe a class of organoarsenic. 6600 Yongen-Jaya - Yumenoshima Secondhand Shop 1125 - 1224 Treasure Name Description Sell Price Location Chipped Glass A damaged wine glass.
----- FIGURES continued Number Page 5-7 Typical tank car with parts identified 5-22 5-8 Tank car unloading station 5-22 5-9 Bonding and grounding of a flammable liquid tank truck and loading rack 5-23 5-10 Compressed inert gas transfer method 5-25 5-11 Fail-safe transfer line for hazardous fluids 5-25 5-12 Fail-safe transfer line inlet and outlet assemblies. 6250 Akihabara - Akiba Electronics Liquid Mercury A shimmering silver liquid metal used to make tools. An icon used to represent a menu that can be toggled by interacting with this icon.
It is required for aerostats to create buoyancy particularly in lighter-than-air aircraft which include free balloons moored balloons and airshipsOnly certain lighter than air gases are suitable as lifting gases. Keep chemical away from heat sparks or flame Keep container closed when not in use Always wear PPE. Oxidizing Gases Liquids Solids.
This category includes various substances presenting a severe fire risk. Sells for a little. Pyrophoric liquid or solid.
The symbol of the flame indicates a potential fire hazard and includes flammable gases liquids aerosols and solids. Substances and mixtures that produce flammable gases in contact with water. Avoid mixing with other chemicals except as directed keep chemical away from contact with skin eyes or clothing Always wear PPE.
Fire hazards posed by water-reactive substances such as alkali metals and metal hydrides by pyrophoric substances such as metal alkyls by strong oxidizers such as perchloric acid and by flammable gases such as acetylene require procedures beyond the standard prudent practices for handling chemicals described here see sections 6C and 6D and should be researched in LCSSs or other. Oxidizer Oxidizing Agent. See Appendix B of the HCS.
Establishing designated glove-only vs. A pyrophoric toxic powder used for ignition. No-glove items such as pens keyboards instruments drawers door handles refrigerators and work spaces.
Use care when handling avoid. SkinEye Corrosion Corrosive to Metals. Self-heating substances and mixtures.
Pyrophoric liquids gasses and solids. A022 The criteria for determining health hazards are test method neutral ie they do not specify particular test methods as long as the methods are scientifically validated. The possible stronger carcinogenicity of low solubility chromate vs soluble chromate compounds may derive from the combinative genotoxic effects of intranuclear CrIII and the persistent exposure to a strong oxidant CrVI.
Flammables Self-heating Emit Flammable Gases Pyrophoric Gases Liquids Solids Organic Peroxides. Do not wear gloves in hallways offices break rooms elevators restrooms or any other public areas. And self-reactive substances and mixtures.
Eye Irritation Skin Irritation SkinRespiratory Sensitization Carcinogenicity Mutagenicity Reproductive Hazards. A023 The term scientifically validated refers to the process by which the reliability and the relevance of a procedure are established for a. NJDOH RTK Hazardous Substance List.
Or in contact with water emits flammable gas. Solids contaminated with flammable liquids. Its flashpoint is 45 C and its autoignition temperature is.
It is a normal part of rocks soil air and water and it occurs in nature in the form of minerals but never as a metal. 60o C. A021 There is no requirement for testing chemicals.
General health hazard Respiratory sensitizer Organ Toxicity. Uranium metal is silver-colored with a gray surface and is nearly as strong as. This category encompasses all flammable gases featuring a red placard and a fire symbol.
Methylenediphenyl diisocyanate MDI Monomethyl -tetrachlorodiphenyl. Inspection repair alteration and reconstruction of steel aboveground storage tanks used in the petrochemical industry Training only Course Instructors. Acute Toxicity - Oral Dermal Inhalation.
A lifting gas or lighter than air gas is a gas that has a lower density than normal atmospheric gases and rises above them as a result. Chem Res Toxicol 18 10. Flammable gases aerosols liquids or solids.
Because it is heavier than air diethyl ether vapors tend to linger more crawl on table tops and could potentially ignite when an open flame working hot plate or spark is nearby. Thin Copper A thin copper coin.
1 mole 602 x 1023 particles 1 mole molar mass could be atomic mass from periodic table or molecular mass. What is the molecular weight of an unknown monoprotic acid if 04955 g of the acid are neutralized by 3700 mL of a 01000 M NaOH solution.

Answered What Mass Of Sodium Hydroxide Naoh Bartleby
Sodium hydroxide NaOH - Sodium hydroxide is an ionic compound.

Molecular mass of naoh. Since the molar mass of NaOH is 40 gmol we can divide the 90 g of NaOH by the molar mass 40 gmol to find the moles of NaOH. Molarity is a measure and unit of concentration. If the equation is arranged correctly the mass units g cancel out and leave moles as the unit.
Binary molecular covalent compounds are formed as the result of a reaction between two nonmetals. 00 amu 3 The molecular mass of H2SO4 98. This the same as multiplying by the reciprocal of 40 gmol.
Of moles Given mass of compound in the questionMolecular mass of the given compound Thanks. Check the chart for more details. 1M solution of NaCl 23Na 35Cl 58 g.
Exposure to Magniosan causes mild irritation in the mucous membranes. Elemental composition of NaOH. Although there are no ions in these compounds they are named in a similar manner to binary ionic compounds.
Considering that the molecular weight of KHP is MWKHP 20423 gmol the concentration of the KHP solution is. HCl does not have a high molecular mass. Therefore heating it results to decompose into magnesium oxide nitrogen oxides and oxygen.
1 u is equal to 112 the mass of one atom of carbon-12. The molecular weight of the substance and the identity of the unknown can than be determined based on the number of moles and the weight of the sample. If you need to prepare roughly one liter of 1 M NaOH solution you dissolve the molar mass of NaOH 400 g using distilled water in a beaker then transfer this solution to a one liter volumetric.
It solidifies at. A unit of concentration equal to the number moles of solute in a 1L of solution. A second stable eutectic composition is 454 mass of NaOH that solidifies at about 49 C into a mixture of crystals of the dihydrate and of the 35-hydrate.
Convert grams NaOH to moles or moles NaOH to grams. Molecular mass NaOH 23 g x 1 16 g x 1 1 g x 1 40 g mol-1. Volume of solution Mass of solution density 100 g 1.
It can be calculated by adding the invididual molar mass of every atom that are composing the molecule CH4. 16 g mol 23 g mol 1 g mol 16 g mol 40 g mol 23 g mol 1 g mol 40 g mole. The result from the above calculation will then be multiplied by the mole ratio of Na_2SO_4 and NaOH which is 1 mol Na_2SO_42 mol NaOH.
Symptoms include shortness of. On the other hand molar mass is a unit of mass. Molecules of CO2 48g Molarity dilutions.
All masses must be to nearest hundredth 1 NaOH 2 H 3 PO 4 3 H 2 O 4 Mn 2 Se 7 5 MgCl 2 6 NH 4 2 SO 4 There are three definitions equalities of mole. 2 MgNO 3 2 2 MgO 4 NO 2 O 2. Not Helpful 16 Helpful 109.
Mass percent composition Atomic percent. Since we are asked to find the mass of Na_2SO_4 formed in this reaction we need to multiply the answer of step 3 to the ratio of the formula mass of Na_2SO_4. OHaq KHPh aq H2O l KPhaq For the highest accuracy a sample size is.
Molar mass of NaOH is 3999711 000037 gmol Compound name is sodium hydroxide. The third stable eutectic has 184 mass of NaOH. These examples show how the rules.
1 moles NaOH 3999711 gram using the molecular weight calculator and the molar mass of NaOH. What are the molecular weights of the following compounds. Amedeo Avogadro is considered to be the number of units usually molecules or atoms in a substance that is proportional.
Substance MW or FW molar mass Fe 55. Potassium permanganate is an inorganic compound with the chemical formula KMnO 4 and composed of K and MnO 4It is a purplish-black crystalline salt that dissolves in water to give intensely pink or purple solutions. The molar mass of a substance also often called molecular mass or molecular weight although the definitions are not strictly identical but it is only sensitive in very defined areas is the weight of a defined amount of molecules of the substance a mole and is expressed in gmol.
H 3 x 1. Assuming your 20 is weight on weight just add 80 grams. The hydrogens atomic mass and number are both 1 while oxygens number is 8 and its mass is 16.
Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u. Do a quick conversion. MgNO 3 2 2 NaOH MgOH 2 2 NaNO 3.
It is a white translucent crystalline solid and used in the manufacturing of detergents and soaps. 2298977 159994 100794. Element Symbol Atomic weight Atoms Mass percent.
Molar mass of NaOH 3999711 gmol This compound is also known as Sodium Hydroxide. Sodium bicarbonate baking soda NaHCO3 can be purified by dissolving it in hot water 60 C filtering toexample. Na O H NaOHa O H NaOH.
2 48gmole 602x10. If you want to find the molar mass you need to know how many grams of acid are in 1 mole or if you knew how many moles were in the sample above then you could calculate the molecular mass. So how many moles of acid are.
How do you make a 20 NaOH solution. Now molecular mass calculator add all masses of substances together. As you will soon see in the technique section of this laboratory this experiment requires that you weigh a certain mass of the unknown salt dissolve it into a solvent and pass it over a column of ion-exchange resin.
Total number of moles n A n B 5 025 525 mol. Number of moles of NaOH n B 10 g 40 g 025 mol. The molar mass of the NaOH compound is 40 gmol.
A hot water solution containing 731 mass of NaOH is an eutectic that solidifies at about 6263 C as an intimate mix of anhydrous and monohydrate crystals. 48149 g 0250 L 00943035 M 943035 mM KHP 20423 gmol c The titration standardization results using 2500 mL aliquots of the KHP solution are summarized in Table 1 below. Definitions of molecular mass molecular weight molar mass and molar weight.
To learn about the structure Properties Preparation Uses Health Hazards and FAQs of Sodium hydroxide NaOH. Visit BYJUS for more information. Examples of molecular weight computations.
Number of moles of water n A 90 g 18 g 5 mol. Latex90text gspace textNaOH times frac1 text mol40text g 225 space textmol NaOH. This gives water a molecular mass of 18Acetic acid.
What is the difference between molarity and molar mass. CO3- because the background. Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
The molar mass of KHPh is 20423 gmole and it has one acidic proton which will react quantitatively with OH. Of any pure substance has a mass in grams exactly equal to that substances atomic or molecular mass mass of one mole of a substance. The molecular weight of sodium hydroxide is 40 gmol.
The nomenclature of binary covalent compounds follows these rules. Has a medium to high molecular weight For this experiment a solution of NaOH which has an approximate concentration of 01 M will be standardized using potassium acid phthalate KHPh. Chemical compound - chemical compound - Binary molecular covalent compounds.
Magnesium nitrate has a high affinity towards water. Compound Moles Weight g. Potassium permanganate is widely used in chemical industry and laboratories as a strong oxidizing agent and also as a medication for dermatitis for cleaning wounds and.
Density of solution 1070 g cm-3. How do you answer this question. It is used to express concentration of a particular solution.
Convert between NaOH weight and moles. Trial 1 Trial 2 Trial 3 Initial volume mL 150 050 2460 Final volume. Mole fraction of NaOH x B n B n A n B 025525 00476.
For the relief of tooth sensitivity and is also used as a pesticide. The name of the element is derived from the Latin words nitron and genes for nitre potassium nitrate forming Elemental nitrogen comprises about 78 of the Earths.

Magnesium Nitrate Facts Formula Properties Uses
The nitrate ion with the partial charges shown.

Magnesium nitrate molecule. Small Molecule Groups Approved Structure. Salts containing this ion are called nitrates. What Is Reduction.
Positive charges indicated by Roman numeral. In the textile. What is the relative mass formula of sodium chloride.
The aldehyde version of this nonanal also causes undergrads some amusement especially when a hyphen is. All group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure. The ammonia can also be in the form of ammonium salts such as ammonium nitrate NH 4 NO 3 ammonium sulfate NH 4 2 SO 4 and various ammonium phosphates.
Its official name is magnesium iron silicate hydroxide and it has the formula. The molecule shown is 2-nonanone but 5-nonanone with the CO group in the middle would be the same forward as well as backwards thus being palindromic in spelling and in structure. The molar mass of magnesium nitrate equals 14833 gmol.
Recently a novel vertical cutoff wall backfill comprised of reactive magnesium-activated slag bentonite and sand referred to as MSBS backfill has been developed by Wu et al. C 6 H 14. Nitrate is a polyatomic ion with the chemical formula NO 3.
An illustration describing the structure of the silver nitrate molecule is provided below. Magnesium deficient sweetpotato leaves become reddish-purple. In the trial subjects took a daily dose of ZMA which included 30 mg zinc monomethionine 450 mg magnesium aspartate and 105 mg vitamin B-6 at night during 7 weeks.
The ion is the conjugate base of nitric acid. Cytokine immune system signaling molecule. Fe 2 ironII ion Fe 3 ironIII ion Cu copperI ion Cu 2 copperII ion b Older but still used rule.
Magnesium is a chemical element with the symbol Mg and atomic number 12. The MSBS backfill is shown to possess excellent unconfined compressive strength hydraulic performance and durability. Nitrogen is a colorless odorless gas.
The chemical formula of table salt or sodium chloride is. NaCl There are one sodium atom and one chlorine atom in each molecule. In one molecule of the compound determine how many atoms of every element are present for each one of these chemical formulas.
3 c magnesium nitrate MgNO. The number of positive charges is not indicated in the name because it is not necessary Rule. Sodium Chloride is an ionic solid with the formula Na Cl-.
Plasma nitrate Ø. The relative mass formula of sodium chloride is M r NaCl A. In its elemental form nitrogen is found as the diatomic molecule N 2 in which the two nitrogen atoms are held together by a triple bond.
A silver metal Ag b ammonia gas NH. Balance oxygen last since it is present in more than one molecule on the right side of the equation Show Answer. Consider as an example the reaction between one methane molecule CH 4 and two diatomic oxygen molecules.
Latin stem for. A 5494 g. Magnesium is a cofactor in protein synthesis muscle relaxation and energy production.
Calculate the molar mass for each of the following substances. Fifty subjects were evaluated using 2 tactile methods and cold air sensitivity dental air syringe along with subjective. Quercetin hesperetin and caffeic acid 5-S-cysteinyl-dopamine and its oxidation product dihydrobenzothiazine.
While any expression that cites the number and kind of atoms is a. Creating firework colors is a complex endeavor requiring considerable art and application of physical science. Carbon dioxide is a one-carbon compound with formula CO2 in which the carbon is attached to each oxygen atom by a double bondA colourless odourless gas under normal conditions it is produced during respiration by all animals fungi and microorganisms that depend directly or indirectly on living or decaying plants for food.
Now the equation is balanced with 2 Chlorides Cl with total charge -2 and 3 Chromiums with total charge 3 on both sides. Write a balanced equation for the decomposition of ammonium nitrate to form molecular nitrogen molecular oxygen and water. Structure for Magnesium hydroxide DB09104.
Na 23 Cl 355 Solution. A manganese metal Mn b sulfur hexafluoride SF. Each molecule contains 2 hydrogen atoms and 1 oxygen atom.
It can be observed that silver has an. Almost all inorganic nitrates are soluble in water. Structure for Potassium nitrate DB11090.
It is sometimes convenient to use. Small Molecule Groups Approved Investigational Structure. Magnesium carbonate MgCO 3 Methanol CH 3 OH Argon gas Ar Dimethyl sulfoxide C 2 H 6 OS Iron iii oxide Fe 2 O 3 CHEMISTRY Related Links.
100951524732 Chemical Formula KNO 3 Synonyms. Other hormones like IGF. Excluding propellants or special effects the points of light ejected from fireworks termed stars generally require an oxygen-producer fuel binder to keep everything where it needs to be and color producerThere are two main mechanisms of color production in fireworks.
Preparation of the metathesis method potassium nitrate ammonium nitrate and potassium chloride raw materials products for potassium nitrate and ammonium chloride recycled mother liquor wherein the device is provided close to the production of a shunt purify production line production line set up in addition to sodium unit and the unit except magnesium which in addition to the sodium. We get Cr 3 2Cl-1 Cr 3 Cl-1 2. There are six C atoms and 14 H atoms in a hexane molecule which has a molecular formula of.
57990521206 Chemical Formula H 2 MgO 2 Synonyms. In a dentifrice or gel to alleviate dentinal hypersensitivity. It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2 or alkaline earth metals of the periodic table.
An example of an insoluble nitrate is bismuth oxynitrate. Note there is no subscript for the number 1 Types of Chemical Formulas. When exposed to PbZn testing liquids with concentrations of 01 mgL 4.
MOL SDF PDB SMILES InChI. HCN hydrogen cyanide Its a toxic gas C 18 H 21 NO 3 codeine a painkilling drug Ca 10 PO 4 6 OH 2 Hydroxyapatite that is present in the enamel of the tooth. The relative mass formula of water is M r H 2 O 2 A r H A r O 2 1 16 18.
Potassium nitrate has been used. PPI PPI PPI Deficiency symptoms Because Mg is a mobile element and part of the chlorophyll molecule the deficiency symp-tom of interveinal chlorosis first appears in older leaves. Some research shows that this combination can help raise testosterone levels.
The aim of this study was to compare a 3 potassium nitrate02 sodium fluoride mouthwash with a 02 sodium fluoride control mouthwash in a 6-week double-blind study. Ammonia is also used in the manufacture of commercial explosives eg trinitrotoluene TNT nitroglycerin and nitrocellulose. Urea H 2 N 2 CO is the most commonly used source of nitrogen for fertilizer worldwide.
This is how the redox equations are balanced. Hno3 Molar Mass. 6 c strontium acetate SrC.
MOL SDF PDB SMILES InChI. Mg 2 magnesium ion H hydrogen ion Sr 2 strontium ion Al 3 aluminum ion Comment. When magnesium hydroxide reacts with nitric acid it produces magnesium nitrate and water MgOH2 2HNO3 MgNO32 2H2O In one to two sentences explain how this reaction demonstrates that matter is neither created nor destroyed in a chemical reaction 2 See answers Advertisement Advertisement Oseni Oseni It can be shown that matter is neither created nor destroyed.
To balance the unbalanced chloride molecule charges we add 2 in front of the chloride on LHS. Leaf tissue between the veins may be yellowish bronze or reddish while the leaf veins remain. Cocoa polyphenols unspecified prostacyclin.
This supplement is a combination of zinc magnesium and vitamin B-6. Calculate the molar mass for each of the following substances. One atom is present in each of the elements hydrogen carbon and.
Nitrates are common components of fertilizers and explosives.
Administer iron products at least 2 hr before and no less than 6 hr after each dose to avoid chelation with magnesium. Fattier meat high in protein.

What Is Sodium Metabisulfite E223 In Food Uses And Safety
Total water intake includes drinking water water in beverages and water that is part of food.

Foods that contain sodium sulfate. Sodium lauryl sulfate SLS is found in shampoos soaps various beauty and cleaning products and even some foods. Today there are many companies which make natural toothpastes free from Sodium Lauryl Sulfate. Typically store-bought muffins contain over 400 calories and a third of the days fat and eating half now and saving the rest for later is near impossiblelikely because foods rich in carbs fat and sugar can be downright addicting.
Leaner protein with a. Most toothpastes contain Sodium Lauryl Sulfate which is a chemical used in toothpaste to create the foaming action. Full content visible double tap to read brief content.
Palm oil is the most widely consumed vegetable oil on the planet found in many packaged products sold in the supermarket. Significant than actual sulfur content of foods. Salatrim short and long chain acyl triglyceride molecule shark cartilage.
Sulfites are chemicals that are in some foods either naturally or as additives. SLS can cause or irritate existing allergies canker sores and bad breath which is why an SLS Free alternative is worth considering. However ferrous gluconate contains less elemental iron than ferrous sulfate so a greater dosage may be needed to correct a deficiency.
While palm oil is the most efficient source of vegetable oil its rapid expansion threatens some of the planets most important and. Typically store-bought muffins contain over 400 calories and a third of the days fat and eating half now and saving the rest for later is near impossiblelikely because foods rich in carbs. Sodium bisulfite okay in wine mead cider sodium cyclamate.
Potassium-rich foods that have an alkalizing effect include fruits vegetables almonds and lentils. Which Everyday Products Contain Palm Oil. Sodium zirconium cyclosilicate will decrease the.
SLES sodium lauryl sulfate SLS ammonium lauryl sulfate ALS and. You might not cook with it but you almost certainly eat or use palm oil. Sodium Lauryl Sulfate commonly known as SLS is a widely used and inexpensive chemical found in many mainstream personal hygiene products such as shampoos toothpastes mouthwashes bodywash soaps detergents and body washThis substance can also go by the name of sodium salt sodium dodecyl sulfate monododecyl ester sulfuric acid and sodium salt sulfuric acid.
Learn more about this chemical READ. This common ingredient creates the foam when you brush but can also irritate sensitive teeth and gums. Celery beets and milk are a few of the foods where youll find it naturally.
Packaged and prepared foods like canned soups lunch meats and frozen dinners often have sodium added during manufacturing either as salt or other common forms of. Fresh fruit and vegetables contain sodium at concentrations in the range. Sodium occurs naturally in some foods and is often added during manufacturing.
Adrenaline Ana-Kit EpiPen epinephrine A-Hydrocort Solu-Cortef hydrocortisone-injectable Amikin amikacin Aramine metaraminol Celestone betamethasone phosphate Compazine prochlorperazine Decadron dexamethasone phosphate Demerol. Help others learn more. Brief content visible double tap to read full content.
906 x 622 x 197 inches. It is a white water-soluble solid that serves as a buffering and chelating agent with many applications in the food industryWhen crystallized from water it forms a hexahydrate but it dehydrates above room temperature. Smoke flavor synthetic sodium acid sulfate.
When a food contains thiols it can cause elevation of sulfur. The concentration of SLS found in consumer products varies by product and manufacturer but typically ranges from 001 to 50 in. Sodium is naturally present in all foods and may be added during food processing.
Disodium pyrophosphate or sodium acid pyrophosphate SAPP is an inorganic compound consisting of sodium cations and pyrophosphate anion. Sodium sulfatepotassium sulfatemagnesium sulfate decreases levels of ferrous sulfate by inhibition of GI absorption. Alpha Lipoic Acid or thioctic acid Chondroitin Sulfate Cysteine DMPS DMSADMSO Epsom.
It does NOT contain paraben and sulfate and of course we never hurt our fur friends. For this reason many Sensodyne toothpastes are formulated without SLS to keep your teeth clean and healthy without causing further sensitivity. Two common additives that contain aluminum include sodium aluminum phosphate and sodium aluminum sulfate which are found in self-rising flours and cheeses as well as in cereal flours respectively according to the FDATake note that both of these additives are Generally Recognized as.
Cereals and cheese may contain as much as 1020 gkg. One theory suggests that a long-term high intake of protein foods such as meats poultry fish dairy and eggs as well as cereal grains may create a condition called low-grade metabolic acidosis due to their high sulfate and phosphate content. Sodium laureth sulfate SLES an accepted contraction of sodium lauryl ether sulfate SLES also called sodium alkylethersulfate is an anionic detergent and surfactant found in many personal care products soaps shampoos toothpaste etc and for industrial usesSLES is an inexpensive and very effective foaming agent.
Plus many commercial muffins are also spiked with waist-widening soybean oil and additives like mono- and diglycerides. It is also more expensive than ferrous sulfate. And human and cows milk.
Package Dimensions. Although a low intake of total water has been associated with some chronic diseases this evidence is insufficient to establish water intake recommendations as a means to reduce the risk of. Such foods include frozen products products that are sufficiently thermally processed to kill pathogenic organisms eg canned foods acidic foods pH 38 and foods in which water activity remains low when sodium is removed eg foods with low water activity due to high sugar content Reddy and Marth 1991.
Good sources of protein include. However people are most exposed to aluminum through additives in highly processed foods. Many toothpastes contain sodium lauryl sulfate SLS.
Stringer and Pin 2005. Bleph-10 sulfacetamide sodium Pred-Forte prednisolone acetate Pred-Mild prednisolone Injectable medications. Sodium lauryl sulfate SLS also known as sodium laurilsulfate or sodium dodecyl sulfate is an anionic surfactant commonly used as an emulsifying cleaning agent in household cleaning products laundry detergents spray cleaners and dishwasher detergents.
For other foods reducing sodium content has the. Most dog foods will contain both but you want to be sure youre choosing only premium meats in your dog food. When foods dont contain a high amount of thiols it is believed the sulfur in these foods stays complexed with methionine and does not significantly raise sulfur levels.
Of course we also add it during cooking and at the table too. 39 out of 5 stars 455 ratings. Water is the largest single constituent of the human body and is essential for cellular homeostasis and life.
Applies only to oral form of both agents. Its rare but some people about 1 in 100 according to the FDA are sensitive to these compounds. Browse our full range of SLS-free sensitive teeth toothpastes for gentle 247 protection.
Newer slow-release forms of iron have been introduced which may help reduce gastrointestinal side effects but they are more expensive and usually contain less. Good Sources Of Protein Protein comes from many sources but some of the best that youll find in dog food comes from a variety of meats.
Unfortunately cannabis extractions made with denatured alcohol are. The type of fuel used to power a vehicle.

Vapor Pressure Of Methanol And Ethanol As A Function Of Temperature Download Scientific Diagram
National Toxicology Program Chemical Repository Database.

Vapor pressure of denatured ethanol. Second denaturing can mean breaking down the three-dimensional structure of a molecule such as a protein is denatured when exposed to heat. Chlorine bleach and alcohol ethanol. A minimum test pressure of at least 1 1 2 times MAWP must be maintained for at least 30 seconds.
First it can refer to any process used to make ethanol unfit for consumption denatured alcohol. Denatured Alcohol SAFETY DATA SHEET 1 3 0 06082012 04142009 Revision. For your BAC to return to zero.
Ethanol is a primary alcohol that is ethane in which one of the hydrogens is substituted by a hydroxy group. Details of the supplier of the safety data sheet. Product and Company Identification Product Code.
Gasoline ethanol E85 diesel bio-diesel natural gas or electricity. Molecular interactions within the native state or assembly are replaced by molecular interactions with aqueous surroundings. At 10000.
We would like to show you a description here but the site wont allow us. Ethanol Denatured 95 vv. Replies 1 Views 5K.
Product Name Ethanol Anhydrous Histological Cat No. ETHANOL reacts violently with acetyl chloride and acetyl bromide Rose 1961. 180 mm Hg at 68 F.
Fuelwater separator A device that separates the water from the fuel in addition. Reacts readily with hypochlorous acid and with chlorine to give ethyl hypochlorite which decomposes in the cold and explodes on. 270 mm Hg at 86 F NTP 1992 National Toxicology Program Institute of Environmental Health Sciences National Institutes of Health NTP.
Thermodyn 1970 2 367-372. Is a licence required. Replies 8 Views 21K.
1 hours at 10000. Dilution - Dilution is when a. In addition MC 312 and DOT 412 cargo tank motor vehicles must.
Mixtures with concentrated hydrogen peroxide form powerful explosives. Monster truck tug-of-war. As the alcohol evaporates the level in the glass should recede.
Related Threads on EthanolAlcohol 75 and isopropyl alcohol75 any difference. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O. A405P-4 Synonyms Grain alcohol denatured.
Pure ethyl alcohol is a non-denatured 200proof ethanol. Monocytogenes to lethal. D4815 Test Method for Determination of MTBE ETBE TAME DIPE tertiary-Amyl Alcohol and C1 to C4 Alcohols in Gasoline by Gas Chromatography.
Water 7921e005 mgL 25 C est water 100E06 mgL 25 C exp Organoleptic Properties. For laboratory and manufacturing use only. Is it safe to use 75 Alcohol with PP and other plastic-made container.
It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. Relevant identified uses of the substance or mixture and uses advised against. It acts as a hydrogen source and reaction medium during the transfer hydrogenation of carbonyl compounds in the presence of sodium hydroxide.
Vapor heat capacities and enthalpies of vaporization of ethanol 2-methyl-1-propanol and 1-pentanol J. I think its methylated spirits. Fragrance extraction refers to the separation process of aromatic compounds from raw materials using methods such as distillation solvent extraction expression sieving or enfleurageThe results of the extracts are either essential oils absolutes concretes or butters depending on the amount of waxes in the extracted product.
Ethanol acid hydrogen peroxide heat or salt had variable ef fects on subsequent exposure of L. Use of the substancemixture. Mixtures with concentrated sulfuric acid and strong hydrogen peroxide can cause explosions.
Details of the supplier of the safety data sheet Emergency Telephone Number CHEMTRECÒ Inside the USA. The major problem when starch is used as raw material for cyclodextrin production is the high viscosity of the reaction system which impedes stirring and contact between the enzyme and substrate. 44600000 mmHg 2000 C.
11 This test method covers the use of automated vapor pressure instruments to determine the total vapor pressure exerted in vacuum by air-containing volatile liquid petroleum products and liquid fuels including automotive spark-ignition fuels with or without oxygenates and with ethanol blends up to 85 volume fraction see Note 1This test method is suitable for testing samples with. TCC 944 C. Uses advised against Food drug pesticide or biocidal product use.
It can be synthesized either from molasses by fermentation or by hydration of ethylene. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a. 231 mm Hg at 25 C.
Denatured Alcohol Manufacturer Information Company Name. Ethanol a primary alcohol is an important raw material for the synthesis of various compounds. 0 sweet potato peel as substrate.
Temperature K A B C Reference Comment. This is why a litre of pure Ethanol costs around 30 while a litre of denatured ethanol will usually cost less than 5. 09122012 X 2 0 3 Instability Special Hazard Health Flammability HEALTH FLAMMABILITY PHYSICAL PPE 1.
D4806 Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Engine Fuel. Recommended Use Laboratory chemicals. This is the same thing that causes brown spots in apples when you drop them.
Ambrose Sprake et al 1975. The impact of. And pure alcohol would evaporate too quickly to effectively kill bacteria or viruses on your skin especially during winter when the air is less humid.
To a certain extent all of these techniques tend to produce. Boric acid Isopropyl alcohol. Replies 5 Views 8K.
Research Triangle Park North Carolina. Aug 20 2019. Merck 11th ed 1989.
PVD stands for Physical Vapor Deposition. Diffusion - Diffusion is the movement of particles from an area of higher concentration to one of lower concentration. All data Notes.
This bitter-tasting chemical does not evaporate away with the alcohol and will remain in the extract making it unsuitable for internal consumption of any kind eating as well as vaporisingsmoking. Coefficents calculated by NIST from authors data. When you unfold a protein or an RNA denature them or separate two strands of DNA melt it or disassemble and melt the ribosome then interior regions become exposed to the surroundings which are mostly water plus ions.
Thickness of stainless steel for tank shell and heads for cargo tanks and portable tanks must be the greater of 635 mm 0250 inch or the thickness required for a tank with a design pressure at least equal to 13 times the vapor pressure of the lading at 46 C 115 F. Gasoline is used in cars boats motorcycles lawn mowers and other engines. I The retest and inspection must be performed by a person familiar with salvage cylinders and trained and experienced in the use of the inspection and testing equipment.
Pressure and pulse electric field PEP technologies. Other means of identification. The cylinder must be examined under test pressure and removed from service if a leak or a defect is found.
Its vapor pressure at 20C is 58. D4953 Test Method for Vapor Pressure of Gasoline and Gasoline-Oxygenate Blends Dry Method D5059 Test Methods for Lead and. Val Tech Diagnostics A Division of LabChem Inc.
159 Air 1 Flash Point. Wheat Grain for Mushrooms.
When iodine I 2 comes in. It would lose effectiveness and would often result in mysterious failures of plates to produce an image.

Methyl Iodide An Overview Sciencedirect Topics
Propyne methylacetylene is an alkyne with the chemical formula The template Carbon is being considered for deletion C H 3 CCH.

Methyl iodide vapors. Ether iodide and bromide salts dust and various organic matters. It is a component of MAPD gasalong with its isomer propadiene allene which was commonly used in gas weldingUnlike acetylene propyne can be safely condensed. In humans acute short-term exposure to methyl iodide by inhalation may depress the central nervous system CNS irritate the lungs and skin and affect the kidneys.
When the negative. Methyl chloride CH 3 Cl has a slight not unpleasant odor that is not irritating and may pass unnoticed unless a warning agent has been added. 192655a Employers must limit an employees exposure to any substance listed in.
Footnotes to Table AC-1 a The Chemical Abstracts Service Registry Number is a designation used to identify a specific compound or substance regardless of the naming system. Cyclohexane Cyclohexanol Isopropyl acetate. Bases dont react with hydrocarbons so it will stay in organic phase 4.
Carry out all designated operations in the hood. These numbers were obtained from the Desk Top Analysis Tool for the Common Data Base and. Pure copperI iodide is white but samples are often tan or even when found in nature as rare mineral marshite reddish brown but such color is due to the presence of impurities.
Toxic vapors formed from mercury vaporization or the burning of mercury containing materials can enter the respiratory system and pass readily into the circulation. 3-26 also support this as the range of cluster size for nanofluid prepared by 0 h of. This means that the compound will not remain in the organic phase 3.
EPAs Integrated Risk Information System IRIS is a human health assessment program that evaluates information on health effects that may result from exposure to. Also the side-products are innocuous. Thus it wont remain in the organic phase.
Volatile chemicals produce vapors readily. Program and regulatory information about this substance including links to EPA applicationssystems statues. Methyl eugenol could be present.
Safety and Health Regulations for Construction. This is so you will be aware of their properties. Vapors may form explosive mixtures with air.
Vapors may travel to source of ignition and flash back. Mg BrC 2 H 4 Br C 2 H 4 MgBr 2. The anodic compartment contains the anolyte solution which includes sulfur dioxide SO 2 iodide I and imidazole needed in the chemical reaction.
-CO2H is carboxylic acid which is an acid and will react with a base. This is a noxious liquid. All of the chemicals described in this section are designated with an asterisk in the text the first time they appear.
Massive acute inhalation exposure to methyl iodide has led to. The PSD results of Fig. 3-29 it can be seen that the highest PDI value was found to be 034 for the nanofluid prepared without ultrasonication 0 h.
Vapor explosion hazard indoors outdoors or in sewers. The amount of Mg consumed by these activating agents is usually insignificant. Are all volatile and possess noxious vapors.
Gases vapors fumes dusts and mists. Methyl iodide is used as an intermediate in the manufacture of some pharmaceuticals and pesticides in methylation processes and in the field of microscopy. Goiter enlarged thyroid gland is caused by iodine deficiency and may lead to retardation in physical sexual and mental development in young people.
We would like to show you a description here but the site wont allow us. Siver bromide and silver iodide are sensitive to light. Methanol or ethanol ROH is usually used as a solvent.
Iodine vapor is intensely irritating to mucous membranes and adversely affects the upper and. Exposure to excessive concentrations is indicated by symptoms similar to those of alcohol intoxication that is drowsiness mental confusion nausea and possibly vomiting. They will spread along ground and collect in low or confined areas sewers basements tanks.
The content containers must be tightly closed after each opening to prevent loss of the drug vapors. Enter the email address you signed up with and well email you a reset link. The IR400 Infrared IR Point Detector is a hydrocarbon gas detector that continuously monitors combustible gases and vapors within the lower explosive limit LEL and provides alarm indication.
Once the Grignard reagent phenyl. 4 Iodine is an essential nutrient required for development and functioning of the thyroid gland. The amount of Mg consumed by these activating agents is usually.
Iodine methyl iodide and 12-dibromoethane are commonly employed activating agents. Acetic Acid Vapors A-Excellent Acetic Acid Glacial A1-Excellent Acetic Anhydride B1-Good Acetone D-Severe Effect Acetone 50 water A-Excellent Acetyl Chloride dry A2-Excellent Acetylene A-Excellent Acrylonitrile A1-Excellent Adipic Acid A2-Excellent Alcohols. All equipment and solutions must be kept completely free of water.
If there are -OH groups the base will react with the groups and form the salt. 192655 - Gases vapors fumes dusts and mists. Addition of small amounts of iodine methyl iodide or 12-dibromoethane.
Iodide in aqueous solution. Case control studies have demonstrated that the chronic inhalation of even low concentrations of mercury 07 to 42 µgm 3 can produce tremors sleep disturbances and impaired cognitive skills in workers 12 32 33. CopperI iodide is the inorganic compound with the formula CuI.
This synthesis begins with the formation of the Grignard reagent from bromobenzene in ether in the presence of crushed magnesium turnings. All electronics are contained within an explosion-proof housing so that the IR400s detector. It features an industry-leading response time of less than 3 seconds even with a splash guard installed.
5 INHALATION HAZARDS. When light strikes a film coated with one of these compounds some of the silver ions revert to the metal in tiny nuclei and the film is developed with a reducing agent which causes more silver to deposit on these nuclei. A necessity for chemical engg studs.
Those substances designated with a P may polymerize. Chemists use 12-dibromoethane because its action can be monitored by the observation of bubbles of ethylene. 224-Trimethyl pentane i-Am yl alcohol Ethyl acetate Miscellaneous solvents.
Most vapors are heavier than air. For more information about the substance you may click one of the links below to take you to the relevant section. Methyl chloride may under certain conditions react with aluminum or.
It is used as an adhesive to close small wounds and hold surgical dressings in topical medications and. The relation of ultrasonication durations on the polydispersity index PDI studied for 50 amplitude sonicator power and reported in Fig. Toluene n-Amyl alcohol Methyl acetate Monochlorobenzene.
It is also known as cuprous iodideIt is useful in a variety of applications ranging from organic synthesis to cloud seeding. Both colour and black and white images have relied on silver since the early days of photography. Occupational Health and Environmental Controls.
Titration Cell Courtesy of Mettler-Toledo In coulometric Karl Ficher titration iodine I 2 is generated electrochemically from iodide I. At room temperature and normal atmospheric pressure vapors escape easily from volatile liquid chemicals.
H 2 SO 4 dil. Phosphoric Acid is an acid-containing four atoms of oxygen one atom of phosphorus and three atoms of hydrogen.

Is Potassium Iodide An Acid Or Base Or Neutral
X 2 aq H 2 Ol HXaq HOXaq The extent of reaction decreases down Group 17.

Potassium iodide strong or weak acid. It is also known as phosphoricV acid or orthophosphoric acid. AgI yellow powder that darkens in light photoactive component of silver-based photographic film Thyroxine 3535-tetraiodothyronine C 15 H 11 I 4 NO 4. Celecoxib increases and arformoterol decreases serum potassium.
An operationally defined group of cyanide species that undergo dissociation and liberate free cyanide when refluxed under weakly acidic conditions pH 45-6. The solution shows acidic nature then it may be an acid salt or salt of weak base and strong acid. Before shipping silver iodide consult with the regulatory requirements of the US Department of Transportation.
It has been more than 20 years since the Stewarts concept of SID was introduced which is defined as the absolute difference between completely dissociated anions and cations. According to the principle of electrical neutrality. Phosphoric Acid is a weak acid with chemical formula H 3 PO 4.
Evaluate for loss of therapeutic effect if medication must be coadministered. Neutralisation is a reaction between an acid and an alkali that forms a salt and water. Note the colour chart on the bottle or package.
Hypochlorous acid is a weak acid and will disassociate according to. 2CrO 4 2-aq 2H aq Cr 2 O 7 2-aq H 2 O. Aqua Regia is the Kings Water this is because it is strong enough to dissolve gold the king of metals.
KOH reacts readily with carbon dioxide to produce potassium carbonate and in principle could be used to remove traces of the gas from air. Weak Acid Dissociable WAD Cyanide. Therefore the combined experimental results and DFT calculations indicate that the oxidation of glucose to gluconic acid and glucaric acid is proceeded mainly via the Path 1.
Potassium hydroxide KOH is a strong base. Hydronium ion is a strong acid but chloride ion is such a weak Brønsted base that it can be ignored in Brønsted acid-base reactions. The pH scale is used to measure acidity and alkalinity.
With iodine it is so small that the acidic and bleaching properties of the solution are not seen in this. Strong Acid Dissociable SAD Cyanide. Salts are odourless and have a salty taste and many are soluble in water.
Coadministration of apalutamide a weak CYP2C9 inducer with drugs that are CYP2C9 substrates can result in lower exposure to these medications. Potassium iodide can block absorption of radioactive iodine by the thyroid gland through flooding the thyroid with non-radioactive iodine and preventing intake of radioactive. CID 24841 Hydriodic acid CID 5462222 Potassium Dates.
Then add a few drops of 05 M K 2 CrO 4. A relatively strong oxidizing agentchlorine can react with a wide variety of compounds. Thiosulphate and Hydrochloric acid.
The flame test on the solid chromate is important for confirmation. HOCl H OCl In waters with pH between 65 and 85the reaction is incomplete and both species HOCl and OCl will be presentHypochlorous acid is the more germicidal of the two. Of particular importance in disinfection is the.
Illustrating its hydrophilic character as much as 121 kg of KOH can dissolve in a single liter of water. For small dry spills of silver iodide collect the material and deposit in sealed. Each VERTICAL drop is about 625 mgs of iodinepotassium iodide 25 mg iodine 375 mg potassium iodide and 2 drops is about 1250 mgs of iodinepotassium iodide 50.
H 2 SO 4 also give good indication about the presence ofacid radicals See Tables 71 and 73. Halogens are highly reactive and can form hydrogen halides metal halides organic halides interhalogens and polyhalogenated compounds. In its liquid form it appears as a clear colourless solution and in its solid form it.
Anhydrous KOH is rarely encountered. However iodine will form an aqueous solution in the presence of iodide ion. Ii Reaction between Potassium Iodate KIO3 and Sodium Sulphite.
The three widely used approaches to acidbase physiology are the HCO 3-in the context of pCO 2 standard base excess SBE and strong ion difference SID. Effect of interaction is not clear use caution. In this case it is best to neutralise the solution with sodium carbonate before testing it for anions.
Study of reaction rates of any one of the following. Discuss halogen compounds and their properties. 1 Nickel IIIchloride potassium phosphate -- Molecular equation.
I Reaction of Iodide ion with Hydrogen Peroxide at room temperature using different concentration of Iodideions. Calculating Number of MGs of Iodine and Potassium Iodide per Drop of Lugols Solution. This occurs with the addition of potassium iodide KI forming a triiodide ion.
The big difference in electronegativity between the H and the F results in a large dipole on an HF molecule with the hydrogen having a partial but sizeable. Indicators are substances that change colour with a change in acidityalkalinity. Weak acid dissociable cyanide.
Gases evolved in the preliminary tests with dil. It is present in teeth and bone and helps in metabolic processes. Besides the energy barrier 209 and 1203 kcal mol.
Red pH 1-3 very acidic solution orange pH 4 to 5 weak acid yellow pH 6 very weak acid green pH 7 neutral blue pH 8 very weak base indigo pH 9 to 10 weak base violet pH 11 to 14 very basic solution. It is prepared by mixing three parts of hydrochloric acid with one part nitric acid but in olden days it is prepared mixing and distilling salts. Potassium Iodide is a metal halide composed of potassium and iodide with thyroid protecting and expectorant properties.
Use 2 drops of Universal Indicator to 10 mL of test solution. Ammoniac and distill at a high temperature to form Aqua Regia. Weak acid dissociable cyanide is determined analytically through weak acid distillation and analysis of liberated free cyanide.
KI white crystals iodine component of iodized salt Hydrogen iodide. For example we can mix two parts niter with one part Sal. Silver iodide may form explosive compounds with sodium potassium acetylene ammonia and hydrogen peroxide.
Consequently hydrochloric acid is represented as H. Like the closely related sodium hydroxide potassium hydroxide reacts. It actually forms quite a strong bond with the hydrogen its stronger than C-C or C-H bonds for example so much so that it doesnt fully dissociate into ions in water hence its a weak acid albeit a very nasty one.
Na2SO3using starch solution as indicator clock reaction. HI colourless gas strong mineral acid Silver iodide. The similar energy barrier suggests that the catalytic system can convert gluconic acid and glucuronic acid into glucaric acid in parallel.
Determination of Iodide 1 Dicofol 1 Dissociation constant of ammonia 1 dissociation of acetic acid 1 eclipse 1 Electroanalytical 1 endpoint 1 Environmental effects of Pesticides 1 Ethanol Test 1 Farmers and workers 1 Free Energy Change 1 gauch and anti 1 gravity filtration 1 Health effects of Pesticides 1. To about 1 mL of solution add 10 drops of 6 M CH 3 COOH. Pale yellow solid hormone essential for human.
Hydrochloric acid which is an aqueous solution of HCl is a strong acid but there are essentially no HCl molecules in hydrochloric acid solution because the above reaction is so extensive. Use solubility rulesactivity tables and tables for strong bases and acids to write the equations. This weak acid provides sufficient hydronium ions to lower the CrO 4 2-concentratiion enough to keep CaCrO 4 and SrCrO 4 in solution but to allow the BaCrO 4 to precipitate.
All three halogens react with water to produce a strong acid HX and a weak acid HOX which has bleaching properties and is an oxidising agent. Silver iodide should be stored in cool dark areas away from the above materials. Common examples include sodium chloride potassium iodide calcium carbonate and copper sulfate.