Isobaric vaporliquid equilibria for the binary systems chloroform ethanol and chloroform 1-ethyl-3-methylimidazolium trifluoromethanesulfonate emimtriflate as well as the vaporliquid equilibria for the chloroform ethanol emimtriflate ternary system have been obtained at 100 kPa using a recirculating still. Its formula can be also written as CH 3 CH 2 OH or C 2 H 5 OH an ethyl group linked to a hydroxyl group and is often abbreviated as EtOHEthanol is a volatile flammable colorless liquid with a.

Physical And Chemical Properties Of Ethanol Download Table
Ethanoic acid also commonly known as the Acetic acid is a two-carbon acid and hence is the second member of the carboxylic acid family after methanoic acid which is a one-carbon carboxylic acid.

Ethanol physical and chemical properties. Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compoundIt is a simple alcohol with the chemical formula C 2 H 6 O. Thermophysical properties of mixtures of ethanol with water and dodecane Excess volume of the mixture of ethanol and water volume contraction Heat of mixing of the mixture of ethanol and water Vaporliquid equilibrium of the mixture of ethanol and water including azeotrope Solidliquid. Information regarding the physical and chemical properties of cyanide is located in Table 4-2.
A chemical property can also be used to distinguish one compound from another. Ethyl Alcohol or ethanol C 2 H 5 OH is the type used in the production of alcoholic beverages. Ethanoic Acid - Structure Uses Physical and Chemical Properties.
Other combinations of functional groups were described previously and significant changes in chemical behavior as a result of group interactions were described eg. Indicates that odor should be detectable near ERPG-1. Ethanol 64-17-5 1800 ppm.
Tritiated water 2-ethoxyethyl acetate diethylene glycol monobutyl ether urea di2-ethylhexyl phthalate 2-ethylhexanol ethyl 3-ethoxypropionate and 2-propoxyethanol using full thickness rat skin and human stratum corneum. However this type of property is not as easy to observe as a. Values are given for liquid at 25 o C 77 o F 298 K and 1 bara if not other phase temperature or pressure given.
1 Fruits such. Alcohol any of a class of organic compounds with one or more hydroxyl groups attached to a carbon atom of an alkyl group. Both binary and ternary systems containing ionic liquid present.
Alcohol is produced by fermentation of yeast sugars and starches. Ethanol - Ethanol also called ethyl alcohol grain alcohol drinking alcohol or simply alcohol is an organic chemical compound. Alcohol or ethanol is the intoxicating agent found in beer wine and liquor.
HC 2 H 3 O 2 is the chemical formula for the organic compound acetic acid. Let us learn about the physical and chemical properties of water. Fill in Table 21.
Most of the common alcohols are colourless liquids at room temperature. LEL 33000 ppm. A glance of earth taken from space will depict it blue.
Indicates value is 10-49 of LEL. The higher alcoholsthose containing 4 to 10 carbon atomsare somewhat viscous or oily and they have heavier fruity odours. Review the information in module 2.
Acetic acid is the least complex carboxylic acid aside from formic acid and is. This is especially important if the alcohol is to be used in foods drugs or cosmetics. For full table with Imperial Units - rotate the screen.
Methyl alcohol ethyl alcohol and isopropyl alcohol are free-flowing liquids with fruity odours. The other three types methyl propyl and butyl alcohol if consumed can result in blindness and death even in relatively small doses. To allow participants to discuss the differences and similarities in the chemical and physical properties of ethanol and gasoline.
Ethanol is an important industrial chemical. Chemical ERPG-1 ERPG-2 ERPG-3. In this case the change in chemical and physical properties resulting from the interaction of the hydroxyl and carbonyl group are so profound that the combination is customarily treated as a distinct and.
It is used as a solvent in the synthesis of other organic chemicals and as an additive to automotive gasoline. Ethanol is a primary alcohol that is ethane in which one of the hydrogens is substituted by a hydroxy group. This page provides supplementary chemical data on ethanol.
However each alcohol is a distinct molecule with its own melting point boiling point reactivity toxicity and other properties. This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. 5 and 6 Matter and Materials.
Alcohols may be considered as organic derivatives of water H2O in which a hydrogen atom has been replaced by an alkyl group. Although a correct and scientifically valid IUPAC name for this acid is ethanoic acid you may also find its common name acetic. Have participants take a few minutes to review the prior information and fill in Table 21.
It has a role as an antiseptic drug a polar solvent a neurotoxin a central nervous system depressant a teratogenic agent a NMDA receptor antagonist a protein kinase C agonist a disinfectant a human metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite. This chapter builds on the chapters about the properties of materials in Gr. If a specific alcohol is mentioned for a project dont make substitutions.
Except where noted otherwise. AIHA 2016 PACs Protective Action Criteria Chemical PAC-1 PAC-2 PAC-3. Also called ethanoic acid acetic acid is a colorless liquid compound that plays a vital role in all biological processes.
NA not appropriate. Some of the highly branched alcohols and many alcohols. In vitro percutaneous absorption studies were carried out for.
Water is the chemical substance with chemical formula H 2 O one molecule of water has two hydrogen atoms covalently bonded to a single oxygen atom. Cyanides form strong complexes with many metals particularly those of the transition series. Solutions of ferrocyanides and ferricyanides can form hydrogen cyanide.
The Physical Property fields include properties such as vapor pressure and boiling point as well. Some of the properties learners encountered in the earlier grades are revisited but now we start placing greater emphasis on how properties that may be desirable in a consumer product may become undesirable properties when that product turns to waste. This blue colour is actually water the major part of.
Chemical physical and thermal properties of ethanol also called alcohol or ethyl alcohol. Table 21 Instructor Directions. Physical properties of alcohols.
One example of such complex formation is the reaction of cyanide with iron in the formation of ferrocyanide and ferricyanide complexes. Its chemical formula is sometimes written as CH 3 COOH or CH 3 CO 2 H to emphasize its atomic organization. The purpose of the studies was to compare the rates of absorption for the two species.
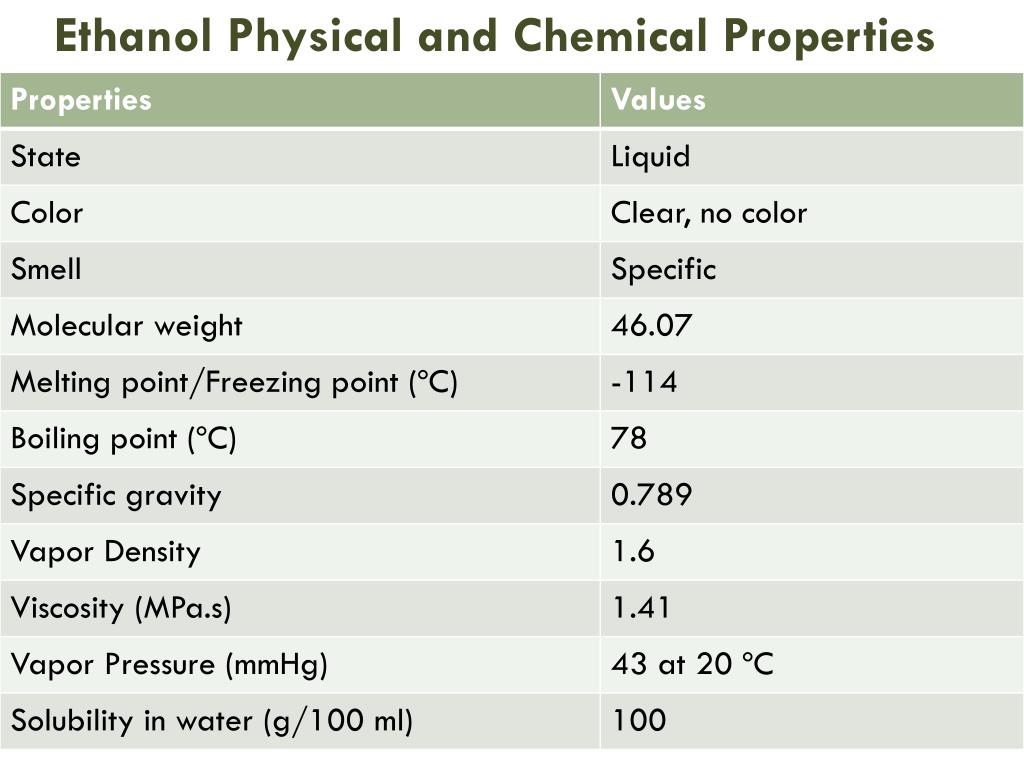
Chemical physical and thermal properties of ethanol.
714 The Chemical And Physical Properties Of Ethanol Water Mixtures Journal Of The Chemical Society Resumed Rsc Publishing

Chemical And Physical Properties Of Ethanol And Gasoline Download Table

Physical And Chemical Properties Of Ethanol Download Table

Property Of Ethanol Molecular Formula C2h50h Ppt Video Online Download
Ppt Ethanol Production Powerpoint Presentation Free Download Id 1950415

Alkene And Alcohols Physical Properties And Chemical Properties And

What Is Ethanol Physical And Chemical Properties Hindi Youtube

Class 10 Science Ethanol Physical Chemical Properties Uses Carbon And Its Compounds Youtube
Ethanol Formula Boiling Melting Point Ph Density Solubility
