According to VSEPR theory Unit 4 methane has a tetrahedral structure Fig. In the following questions two or more options.
Draw the structure of the product formed when the following compound is heated in aqueous base.

Propan-1-ol structure. Why is the reactivity of all three classes of alcohols with cone. Thus R O R is polar. Like water alcohols are polar containing an unsymmetrical distribution of charge between the oxygen and hydrogen atoms.
Marking criteriaNasienriglyne Whole structure correctHele struktuur korrek. Alcool propylique n-propanol 1-propanol N o CAS. 0 Explain why more of isomer 6.
14 14 IBG. Dot. Simple distillation can help in separating a mixture of propan-1-ol boiling point 97C and propanone boiling point 56C.
IUPAC Name propan-1-ol. Give the systematic name of a compound of formula C 5H 10O 2 that gives propan-1-ol as one of the products when it is warmed with aqueous sodium hydroxide. Reagent for Reaction.
Do not write outside the box. Why is it so important to make sure you decant all of the isopropanol supernatant before. 173 Simple Molecular Structures.
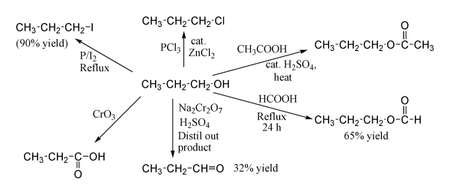
Turn over for the next question. 16 5 Ionic compounds. The oxidation of propan -1 -ol can form propanal and propanoic acid.
Propan-1-ol butan-1-ol butan-2-ol pentan-1-ol i Propan-1-ol butan-2-ol butan-1-ol pentan-1-ol ii Propan-1-ol butan-1-ol butan-2-ol pentan-1-ol iii Pentan-1-ol butan-2-ol butan-1-ol propan-1-ol iv Pentan-1-ol butan-1-ol butan-2-ol propan-1-ol. Alcohols R-OH take the suffix -ol with an infix numerical bonding position. In alkanes tetrahedra are joined together in which C-C and C-H bond lengths are 154 pm and 112 pm respectively Unit 12.
If higher precedence functional groups are present see order of precedence below the prefix hydroxy is used with the bonding position. 3 2 marks E than isomer Fis formed in this reaction. Propionaldehyde is catalytically hydrogenated to produce 1-propanol.
Methoxyethane is also an isomer of C. Therefore the polarity of two R O groups does not get cancelled and these have net dipole moment. The chemical structure C 3 H 8 O exists as several isomers of propanol as well as the isomer methoxyethane.
The reaction between C 2 H 5 COOH and C 2 H 5 OH produces an ester called as propyl ethanoate. Aqueous phases and for all the precipitation reactions. Boiling point C.
12-dichloropropane 4 6. Liquids with a difference of more than 20C in their boiling points can be separated by simple distillation. The two propanol isomers consist of propan-1-ol and propan-2-ol also known as isopropyl alcohol which are distinguished by the placement of an oxygen atom either on the terminal carbon atom or the central carbon atom respectively.
When the acids form salts this is lost and replaced by a metal. The structure of a compound is shown. Apparence liquide incolore limpide dodeur caractéristique 1.
Ethane and propan-1-ol C. Octan-1-ol has higher density and a higher boiling point than propan-1-ol. The structure of E is shown.
Assertion A. 13 13 Turn over IBGJun1974042 Do not write outside the box2. Propan-1-ol and octan-1-ol both have a single OH group and have both hydrogen bonding and dispersion forces as intermolecular attraction.
Propanol is one of the most common types of alcohol. Assume that an excess of oxidising agent is used. Hence the carbonyl carbon is an electrophilic Lewis acid and carbonyl oxygen a nucleophilic Lewis base centre.
Give the structure of Compound. Octan-1-ol has stronger intermolecular bonding due to its molecules having a larger hydrocarbon tail than propan-1-ol. 2 marks Structure.
13C NMR spectroscopy 13C NMR spectroscopy measures the difference in energy between the aligned spin state and unaligned spin state The. Ethers have structures similar to water and have angular or bent structure. Dont draw a line between the two implying a covalent bond.
The high electronegativity of the oxygen. C NMR spectrum is shown. 171 Formation of Covalent Bonds.
Journals books. News. Ii Ethylmagnesium chloride to propan-1-ol iii Propene to propan-2-ol.
Propanol and propanoic acid D. Draw the structure and name the product formed if the following alcohols are oxidized. This geometrical arrangement reflects the effect of electron repulsion and the increasing steric bulk of the substituents on the central oxygen atom.
Carbonyl compounds have substantial dipole moments and are polar than. 0 6 5 marks. The structure of an alcohol is similar to that of water as it has a bent shape.
The suffixes -diol -triol -tetraol etc are used for multiple -OH groups. HCl and ZnCl 2 Lucas reagent different. Propionaldehyde is made by hydroformylation ethylene.
Name of mechanism. The boiling points of these compounds are shown in. Structure properties spectra suppliers and links for.
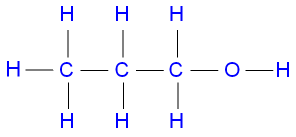
1-Propanol is a primary alcohol with the formula CH 3 CH 2 CH 2 OH and sometimes represented as PrOH or n-PrOHIt is a colorless liquid and an isomer of 2-propanolIt is formed naturally in small amounts during many fermentation processes and used as a solvent in the pharmaceutical industry mainly for resins and cellulose esters and sometimes as a disinfecting agent. Propanol has the formula CH 3 CH 2 CH 2 OH. Suggest the reagent for Reaction.
1 Name and outline the mechanism for the formation of E in this reaction. 131 in which carbon atom lies at the centre and the four hydrogen atoms lie at the four corners of a regular tetrahedron. The bond between the sodium and the ethanoate is ionic.
Jump to main content Jump to site nav. All H-C-H bond angles are of 1095. 1212 Structure of the Carbonyl Group π Fig121 Orbital diagram for the formation of carbonyl group The carbon-oxygen double bond is polarised due to higher electronegativity of oxygen relative to carbon.
I CH 3 CH 2 CH 2 CH 2 OH ii 2-butanol iii 2-methyl-l-propanol Delhi 2012 Answer. 1-Propanol Propan-1-ol 71-23-8 71-31-8 109-78-4 927-74-2 36294-23-2. C 3 H 8 O Isomères Masse molaire 3 gmol C 5996 H 1342 O 2662 pKa.
Multiple Choice Questions Type-II Note. I Both A and R are correct and R is the correct explanation of A. Option i is the answer.
Bonds Structure. 2 2 Only functional group correct Slegs funksionele groep korrek Max. 40 35 30 25 20 Chemical shift ppm 15 10 5 0 Which compound gives rise to this spectrum.
What is the IUPAC name for isopropyl alcohol. 1023 g of propan-1-ol M 6011 g mol-1 was burned in a spirit burner and used to heat 200 g of water in a copper calorimeter. 1 mark Draw the structure of F.
NMR N M R u c l e a r a g n e t i c e s o n a n c e MRI magnetic resonance imaging 13C NMR spectroscopy. Sodium ethanoate for example has the structure. Br Br C F H C H OH C H Br What is the preferred.
Ethylene glycol CH 2 OHCH 2 OH is ethane-12-diol. Any correct structure of. Very useful for determining the structure of unknown compounds Shows the different magnetic environments of carbon atoms in a compound.
CH 3 CH 2 CH 2 OH is propan-1-ol. Propan-1-ol Structure du propan-1-ol. Membership.
174 Giant Covalent Structures. Assume that an excess of oxidising agent is used. Propan-1-ol n-propyl alcohol 1-propyl alcohol or n-propanol are all names for this colourless oil.
Depending on whether or not you wanted to stress the ionic nature of the compound this would be simplified to CH 3 COO-Na or just CH 3 COONa. Atomic Structure TOF - SCT Page 2 of 17 d In a TOF mass spectrometer the time of flight t of an ion is shown by the equation In this equation d is the length of the flight tube m is the mass in kg of an ion and E is the kinetic energy of the ions.
Difference Between Propan 1 Ol And Propan 2 Ol Definition Properties And Structure Similarities And Differences
Gcse Chemistry What Are The Isomers Of Propanol What Is A Primary Secondary Or Tertiary Alcohol Gcse Science

File Propan 1 Ol 2d Flat Png Wikimedia Commons

1 Propanol Purum 99 Gc Honeywell 1 Propanol Solvents Fisher Scientific

How To Draw The Structure For Propanol Or Propan 1 Ol Drawing Alcohol Structures Chemistry Youtube

File Propan 1 Ol Lewis Svg Wikimedia Commons