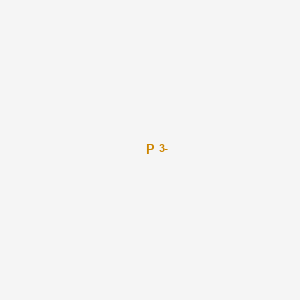Ionic compounds are compounds made up of ions. Copper II bromide CuBr2 8.

How To Write The Formula For Iron Iii Phosphide Youtube
Sign up and fill out a short form to start offering your service.
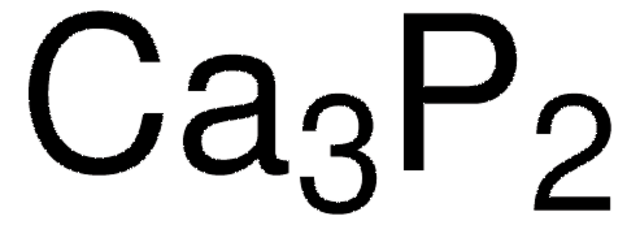
Formula for phosphide. PO 4 3-Æ HPO 4 2-Æ H 2PO 4-phosphate hydrogen phosphate dihydrogen phosphate. Many different phosphides are known with widely differing structures. Binary Ionic Compounds Type II The cation of a transition metal is always named first like any cation and the anion second.
Copy this to my account. Use Lewis dot structures to show the covalent bonding in the following pairs of. Which element comes first in the name and formula of the compounds in Model 2 or the nonmetal.
In chemistry a phosphide is a compound containing the P 3 ion or its equivalent. OptixeXpert is our new website that aims at connecting optical professionals with employers looking to outsource optics related work. 11 tetraphosphorus triselenide P4Se3.
Ba3N2 __barium nitride__ Na3P ___sodium phosphide Exercise. IronII chloride or ferrous chloride The cation charge must be specified since iron can form more than one charge. E-mail to a friend.
10 N2O3 dinitrogen trioxide. Those materials consisting only of phosphorus and a less electronegative element. Numerous are polyphosphides which are solids consisting of anionic chains or clusters of phosphorus.
P 10 S 4. Ionic Compounds Naming and Formula Writing. Write the correct chemical formula for the ionic compound that forms.
3 aluminum oxide Al and O 6 magnesium phosphide Mg and P Formula. Lead II chloride PbCl2 5. Metals combine with polyatomic ions to give ionic compounds.
Cesium phosphide __Cs3P__ calcium iodide _CaI2_ barium fluoride __BaF2__ magnesium nitride __Mg3N2__ aluminum bromide __AlBr3__ sodium selenide _Na2Se. Aluminum Al 3 barium Ba 2 bismuth Bi 3 cadmium Cd 2. Write the formulas of the following chemical compounds.
13 iron II phosphide Fe3P2. In the compound zinc phosphide what is the. Most commonly encountered on the binary phosphides ie.
The positive charge more protons versus electrons for a cation is shown by a number and plus sign after the formula. 1 barium oxide Ba and O 4 sodium oxide Na and O Formula. Zinc iodide ZnI2 3.
I Calcium phosphide ZnCO 3 I Zinc carbonate NaNO 3 I Sodium nitrate H 2 SO 3 A Sulfurous acid AgCl I Silver chloride CH 4 M Carbon tetrahydride Cu 2 C 2 O 4 I Copper I oxalate SnI 4 I Tin IV iodide PbS 2 I Lead IV sulfide CO 2 M Carbon dioxide Al 2 SO 4 3 I Aluminum sulfate. Hg23P2 mercury I phosphide Section D Write the formula for the ionic compounds containing transition metals BE CAREFUL TRANSITION METALS MAY HAVE ROMAN NUMERALS and NICKNAMES 1. An ionic compound is formed by the complete transfer of electrons from a metal to a nonmetal and the resulting ions have achieved an octet.
Sodium chloride NaCl and magnesium oxide MgO. Metals lose electrons to produce positve ions called cations. Formula Type Chemical Name HCl A Hydrochloric Acid Hg 2 SO 4 I Mercury I sulfate N 2 O 3 M Dinitrogen trioxide CdS I.
Use the table of ions in Model 1 to answer the following questions. Type II Metal Non-Metal In general it is NOT possible to use the. Optical constants of CaCO 3 Calcium carbonate Calcite Ghosh 1999.
Copy this to my account. Name Formula Systematic Name Common Name Formula Name Formula Methane CH 4 Methanoic acid Formic acid HCO 2H 12-Dichloroethane C 2H 4Cl 2 Ethane C 2H 6 Ethanoic acid Acetic acid CH 3CO 2H Methylamine CH 3NH 2 Propane C 3H 8 Propanoic acid Propionic acid C 2H 5CO 2H Methylammonium ion CH 3NH 3 Butane C 4H 10 Butanoic acid Butyric acid C 3H 7CO. 2-then to get the formula for hydrogen sulfate ion you add a hydrogen ion to the front of the formula.
P 4 S 10. Cadmium sulfide CdS 2. 12 potassium acetate KC2H3O2.
7 VO2 vanadium IV oxide. Since a hydrogen ion has a 1 charge the net charge on the new ion is less negative by one. The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator.
14 disilicon hexabromide Si2Br6. 2 calcium chloride Ca and Cl 5 sodium nitride Na and N Formula. This activity includes every compound formula and name that can be formed from the list 44 Ions provided in Chemistry A at Pickerington High School Central.
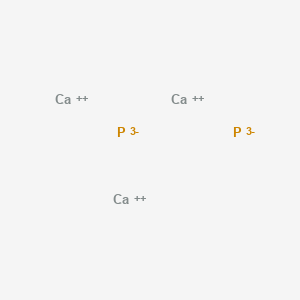
Binary Ionic Formula Practice Name_____ 1 sodium chloride Na1 Cl-1 NaCl 2 lithium bromide Li1 Br-1 LiBr 3 magnesium flouride Mg2 F-1 MgF2 4 potassium oxide K1 O-2 K2O 5 calcium sulfide Ca2 S-2 CaS 6 aluminum iodide Al3 I-1 AlI3 7 barium bromide Ba2 Br-1 BaBr2 8 aluminum sulfide Al3 S-2 Al2S 3 9 calcium phosphide P-3 Ca2 P-3. What is the. E-mail to a friend.
Iron III oxide Fe2O3 4. Zinc carbonate ZnCO 3 aluminum hypochlorite Aℓ C ℓO 3 calcium phosphate Ca 3PO 4 2 cadmium phosphate Cd 3PO 4 2 iron III sulfate Fe 2SO 4 3 mercury II chlorite HgC ℓO 2 2 potassium phosphite K 3PO 3 magnesium. Potassium phosphide K 3P zinc carbide Zn 2C manganese IV sulfide MnS 2 cobalt II bromide CoBr 2.
What is the correct molecular formula for the compound tetraphosphorus decasulfide. K 4 S 10. Circle the symbol for the metal in each of the compounds in Model 2.
N Zinc phosphide Al Aluminum oxide Strontium chloride Sr 4. Name the cation first specifying the charge if necessary then the polyatomic ion as listed in the table. It was designed by Alfred Stock 1876-1946 a German chemist and first published in 1919.
The transfer of electrons between metals and non-metals produces charged particles called ions. If theres just a plus sign it means the charge is plus 1. Gallium phosphide The template Gallium is being considered for deletion Ga The template Phosphorus is being considered for deletion P a phosphide of gallium is a compound semiconductor material with an indirect band gap of 224 eV at room temperature.
Ionic Compounds Containing a Metal and a Polyatomic Ion. Using this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Na Mg 2 Non.
Se 4 F. Formula 21 sodium phosphide Na 3 P 22 magnesium nitrate MgNO 3 2 23 lead II sulfite PbSO 3 24 calcium phosphate Ca 3 PO 4 3 25 ammonium sulfate NH 4 2 SO 4 26 silver cyanide AgCN 27 aluminum sulfide Al 2 S 3 28 beryllium chloride BeCl 2 29 copper I arsenide Cu 3. For example Cu is called CopperI and Cu 2 is called CopperII in the names of compounds containing these ions.
Iron II fluoride FeF2 7. History- The type of naming you will learn about is called the Stock system or Stocks system. Below is a chemistry quiz on ionic compounds names and formulas give it a shot and see if you understood all we covered in this topic on Ions.
Undoped single crystals are orange but. P 2 S 5. Ionic Compound Naming and Formula Writing List 1.
What is the correct molecular formula for the compound selenium tetrafluoride. 6 IO2 iodine dioxide. Give formulas for the following compounds refer to periodic table only.
A monatomic meaning one-atom cation takes its name from the name of the element. Zinc phosphide 29 SrC 2 H 3 O 2 2 strontium acetate 30 Cu 2 O copper I oxide 31 Ag 3 PO 4 silver phosphate 32 YClO 3 yttrium I chlorate 33 SnS 2 tin IV sulfide 34 TiCN 4 titanium IV cyanide 35 KMnO 4 potassium permanganate 36 Pb 3 N 2 lead II nitride 37 CoCO 3 cobalt II carbonate 38 CdSO 3 cadmium sulfite 39 CuNO 2. Ag Silver.
Impure polycrystalline material has the appearance of pale orange or grayish pieces. 8 PbS lead II sulfide. The name or symbol of the metal comes first.
Write the chemical formula for the following ionic compounds. An ionic compound is composed of a metal and a non-metal. Magnesium nitride Mg3N2 6.
5 Ag3P silver phosphide. Review some examples of cations or positive ions.
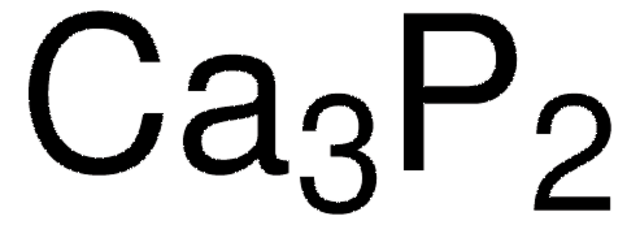
Calcium Phosphide 8 Active Phoshor P Basis 1305 99 3
State The Valency And The Formula Of The Following Radicals I Phosphide Ii Plumbous Iii Mercuric Iv Manganate V Silicate Chemistry Topperlearning Com Qecw66e22

How To Write The Formula For Strontium Phosphide Youtube

How To Write The Formula For Mercury Ii Phosphide Youtube
Calcium Phosphide Ca3p2 Ca3p2 Pubchem

How To Write The Formula For Sodium Phosphide Youtube
Aluminum Phosphide Alp Chemspider

How To Write The Formula For Aluminum Phosphide Youtube