Hydrogen chloride and hydrogen cyanide Tips Acids are always aqueous. 1990-1998 Associate Professor Iowa State University 1988-1990 Associate Professor of Chemistry and Director of Freshman.
The potential does vary with temperature but between 10 40C can be estimated by the equations see reference 2.
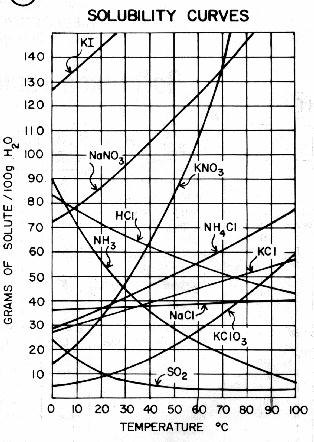
Naoh solubility vs temperature. The solvent that has a high solubility of solute and low solubility of carrier liquid is the ideal solvent that must be chosen in liquid- liquid extraction process. Where T is the temperature C and E. 1998-2013 Professor of Chemistry Iowa State University.
The solubility of benzoic acid in water at 95 oC is 68 gL. By signing up youll get. If the DS is 250 ml but in the range of 250 to 1000 ml in.
Leave the thermometer in the water as you go on to the next step. The pK a for acetic acid is 476. This is consistent with the weakly basic character of LiOH in solution indicating that the Li.
With the increase in temperature stripping efficiency increases. To remove soluble impurities. Temperature is one of the most important parameters in the fermentation and stripping processes.
50 cal cm312. In maceration whole or boorishly powdered plant material is kept in contact with the solvent in a stoppered container for a given period with repeated agitation until soluble matter is dissolved at room temperature for a period of 3 days Handa et al 2008. Example of K a vs.
The process has the purpose to soften and break the plants cell wall to release the soluble phytochemicals. Any phrases that refer to being dissolved or in solution means the compound is aqueous. NaOH CO2 ----- NaHCO3 156 D.
The K a for acetic acid is 175 10 5. The synthesis of polyolefin DRAs is based on low-temperature ZieglerNatta ZN polymerization of higher α-olefins. It is therefore necessary to adjust the stripping temperature to.
LiOH however has a layered structure made up of tetrahedral Li OH 4 and OHLi 4 units. PRECIPITATIVE GRAVIMETRIC ANALYSIS Precipitative gravimetric analysis requires that the substance to be weighed be readily removed by filtration. 047 0015 cal g C.
Viscosity cP at 25C See Figure 4 20. In this article we will explain the electronic configurations ionization enthalpy hydration enthalpy and atomic ionic radii and other physical and chemical properties of the group one alkali metals. Ultra-high molecular weight poly-α-olefins are widely used as drag reducing agents DRAs for pipeline transportation of oil and refined petroleum products.
E 205 073 T 25 for an electrolyte of 35 M KCl E 199 101 T 25 for an electrolyte of saturated KCl. Note that the relation between the solubility and the solubility product constant depends on the stoichiometry of the dissolution reaction. Measure the temperature of the warm water in the Styrofoam cup and record this value in the data table.
RECRYSTALLIZATION Briefly describe how soluble impurities are separated from the desired compound at the molecular level. 4353 dynes cm. 130 cal cm312.
It is a white solid ionic compound consisting of sodium cations Na and hydroxide anions OH. Vapor pressure at 25C See Figure 1 0600 mm Hg. What is the minimum amount of water in which one gram of benzoic acid can be dissolved at 95 oC.
Stronger acid larger K a smaller pK a. Magnesium hydroxide MgOH 2 is. Immediately after recording the temperature add the equivalent of a handful of ice cubes to the warm water.
Sodium hydroxide is a highly caustic base and alkali that decomposes proteins at ordinary ambient temperatures and may cause severe chemical burnsIt is highly soluble in water and readily. Show all calculations for full credit. The solubility in water of the other hydroxides in this group increases with increasing atomic number.
The room-temperature form of NaOH has the thallium iodide structure. 2015present Senior Instructor II University of Oregon. Solubility in Chemistry refers to the ability of a substance to be combined with another substance.
Surface tension at 20C. 2018 compared NaOH KOH and LiOH solution respectively at a concentration of 7 wv under the temperature ranging between 8C and 20C for rice straw pretreatment the highest lignin removal of 6322 was observed after LiOH pretreated at 15C for 3 min. 80 ca l cm312 Hydrogen bonding.
Elements hydrogen nitrogen oxygen fluorine chlorine ammonia carbon monoxide and carbon dioxide nitrogen monoxide and nitrogen dioxide sulfur dioxide and sulfur trioxide hydrogen-compounds eg. RECRYSTALLIZATION Draw a solubility y vs temperature x plot showing the three common solubility behaviors and indicate which one is that of a good recrystallization solvent. At room temperature 25 oC the solubility of benzoic acid in water is 34 gL.
Do not allow any. The mixture then is. The relationship is below.
For this reason it is meaningless to compare the solubilities of two salts having the formulas A 2 B and AB 2 say on the basis of their K s values. The use of. 90 cal cm312 Polar.
Whereas a DS 250 ml in aqueous media indicates solubility issues in the corresponding GI segments a DS 250 ml at all pH values of interest indicates that dissolution is very unlikely to limit drug absorption. High solubility at high temps and low solubility at low temps. Weaker acid smaller K a larger pK a.
The important thing to note is that the pK a scale is opposite that of the K a scale. Learn its full definition its properties and the different factors that affect solubility. Room-temperature acetylene electroreduction then occurs at a layered double hydroxide LDH-derived copper electrocatalyst which offers an onset potential of.
2013-2014 Visiting Lecturer University of Oregon. 20132015 Morrill Professor Iowa State University. Alkali metals belong to the s-block elements occupying the leftmost side of the periodic tableAlkali metals readily lose electrons making them count among the most reactive elements on earth.
Typically the deposition was performed in a standard two-electrode configuration at room temperature with an electrolyte of 20 M NH 4 Cl and 01 M NiSO 4. Looking at the table at the top of this article the. If you have 155 mL of a 0762 M FeCl3 solution how many grams of FeCl3 are contained in this sample.
Department of Geology and Geophysics University of Utah Salt Lake City Utah 84112 USA. The pK a value is more convenient. Thus the solubility is 88 times 105.
2006 Visiting Professor University of Arizona. 1-Hexene based DRAs the most effective at room temperature typically lose DR activity at low temperatures. Phillipsite and Al-tobermorite mineral cements produced through low-temperature water-rock reactions in Roman marine concrete Marie D.
Be very careful not to add any cold water melted ice in the 400-mL beaker to the warm water. The highest ethanol extraction efficiency of 964 was at 75 C but this temperature has a negative effect on microorganism growth and increases the energy costs. Which of the following ions always form soluble ionic compounds_ select all that apply.
Subsequent to the solubility experiments the dosesolubility ratio DS should be calculated according to the BCS. Specific heat at 295C. In order for a non-filtrable precipitate to form it must be supersaturated with respect to its solubility product constant.
Search for other works by this author on. Sodium hydroxide also known as lye and caustic soda is an inorganic compound with the formula NaOH. In dilute solution at equilibrium the concentration of.
Besides the solvent which nonreactive with the other chemical involved in the extraction and has high boiling temperature are suitable for liquid-liquid extraction process.

Concentration Of Naoh To Dissolve Glass Chemistry Stack Exchange

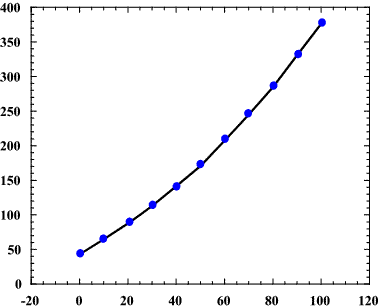
Boiling Temperature At Atmospheric Pressure Of Aqueous Solutions Of Download Scientific Diagram

Solubility Of Naoh In Organic Isopropanol Solvent At Room Temperature Download Scientific Diagram

How Temperature Influences Solubility Chemistry For Non Majors

Solubility Curves Of Sodium Salts Download Scientific Diagram