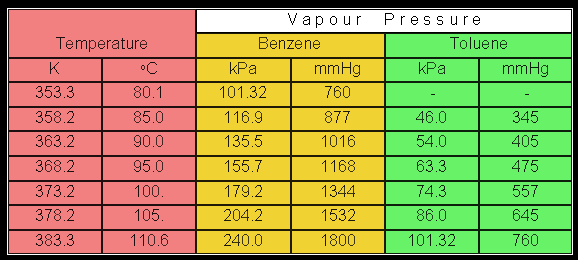
Question from Student Questionschemistry. Vapour pressures of benzene hexafluorobenzene and naphthalene The Journal of Chemical Thermodynamics 1990 22 6.
Vapor Pressure Of Benzene From Dortmund Data Bank
Temperature K A B C Reference Comment.

Vapour pressure benzene. The solubility of naphthalene is closest to ideal for solvents that are similar to it eg. Benzol Phenyl hydride Benzene and mixtures having 10 benzene or more IBC code Name of the supplier. Solution A has vapour pressure of 7 5 4.
Phenylformic acid E210 Sodium benzoate E211 3113. 5 mm of Hg at the normal boiling point of water and solution B has the same vapour pressure at the normal boiling point of benzene. 65-85-0 200-618-2 Sodium benzoate.
However it has threetarget organs central nervous system kidney and hematopoietic system. Vapour Pressure of a Solute above a Solution Henrys Law. Heating the molecules of the liquid can help change them to the vapour phase and thus increase the vapour pressure of the liquid.
Vapour cartridge or canister may be permissible under certain circumstances where airborne concentrations are expected to exceed exposure limits. Temperature and Pressure. If the liquid is heated a little over 100 C the transition from liquid to gas will occur not only at the surface but.
So theres 566 mols of molecules for every 1L of solution one comes from glucose and 556 from water as calculated. The apparent enthalpy of fusion is different and the plot may become curved. The ebulliometric method of vapour-pressure measurement.
It has 6 carbon atoms joined in a ring and has 1 hydrogen atom attached to each of the carbon atoms. Q-Suggest two materials other than hydrogen that can be used as fuels in fuel cells. Lead is also a specific organ toxin.
Vapour may accumulate in hazardous amounts in low-lying areas especially inside confined spaces resulting in a toxicity hazard. Ammonia - Vapour Pressure at gas-liquid equilibrium. P A P A 0 x A and P B P 0 B x B as the total vapour pressure P A 0 x A P 0 B x B is greater than what it should be according to Raoults Law.
Identification SDS Record Number. Protection provided by air-purifying respirators is limited. Trade names and abbreviations 3114.
The pressure lowering of the water is PX as P stands for the pressure of pure solvent and X is the molar fraction of the solute. 1L of water has 1000g of water so there are 100018 mols of water 556 mols. Of solutions say vapour pressure with the concentration of the solution and quite useful in describing the calculations involving gas mixtures.
PCS 95004 Date of SDS. The concentration of a vapor in contact with its liquid especially at equilibrium is often expressed in terms of vapor pressure which will be a partial pressure a part of the total gas pressure if any other gases. At 100 C and atmospheric pressure equilibrium is not reached until the air is 100 water.
Online calculator with figures and tables showing density and specific weight of ammonia for temperatures ranging -50 to 425 C -50 to 800 F at atmospheric and higher pressure - Imperial. There are two effects when the difference between solute and solvent increases. It may be chemical physical or biological in.
CAS EINECS ELINECS number CAS EINECS Benzoic acid. The vapour pressure of the liquid increases with an increase in its temperature. Its quite an experience hearing the sound of your voice carrying out to a over 100 first year.
While naming the substituted benzene compounds we prefix the name of the substituent to the word benzene. 50 g of urea NH 2 CONH 2 is dissolved in 850 g of water. Structural formula Benzoic acid Sodium benzoate.
Benzene Product Description. Benzoic acid Benzene carboxylic acid. Glucose or sucrose are soluble in water but cyclohexane or benzene simple six membered ring compounds are insoluble in water.
The former results from. New Movies to Watch with Your Family this Thanksgiving. Temperature and Pressure - Online calculator figures and table showing density and specific weight of benzene C 6 H 6 at temperatures ranging from 5 to 325 C 42 to 620 F at atmospheric and higher pressure - Imperial and SI Units.
Figures and table showing ammonia saturation pressure at boiling point SI and Imperial units. 5 Toxicology Toxic agent or substance Toxic agent is anything that can produce an adverse biological effect. Common VOCs include ethanol formaldehyde benzene toluene and xylene.
Q-Vapour pressure of pure water at 298 K is 238 mm Hg. This percentage increases as the temperature goes up. What is the mole ratio of benzenepB150 torr and toluenepT50 torrin vapour phase if the given solution has a vapour pressure of 120 torr.
A solution of liquid toluene dissolved in liquid benzene has a benzene mole fraction of 850. Assume that we have 100 g of solution one can start with any amount of solution because the results obtained will be the same. Outdoor VOCs are usually emitted from industrial facilities and vehicles and.
Benzene is a hydrocarbon with the chemical formula C 6 H 6. Calculate the vapor pressure of the solution given that the vapor pressures of pure benzene and toulene are 183 mmHg and 592 mmHg respectively. 11 February 2015 Identity of the substance.
For example Acetone and benzene have higher vapour pressure than water at a particular temperature. Coefficents calculated by NIST from authors data. Benzene is a specific organ toxin in that it is primarily toxic to the blood-forming tissues.
In thermodynamics and chemical engineering the vaporliquid equilibrium VLE describes the distribution of a chemical species between the vapor phase and a liquid phase. VOCs are of concern to both indoor air quality and outdoor quality. Ammonia Gas - Density vs.
Chemistry 38 Calculate the mole fraction of ethylene glycol C 2 H 6 O 2 in a solution containing 20 of C 2H6O2 by mass. In this article we. Assuming X undergoes partial dimerization in benzene what is the percentage of X dimerized in benzene solution.
These liquids will show positive deviation when Raoults Law when. Petrochemical Corporation of Singapore. Use a positive-pressure air-supplied respirator if there is any potential for uncontrolled.
By replacing one or more of the hydrogen atoms with some functional group we get several benzene compounds. Liquid can accumulate static charge by flow splashing or agitation. The slope of the plot changes ie.
Take A Sneak Peak At The Movies Coming Out This Week 812 New Movie Releases This Weekend. Benzene SDS Page 1 of 11 SAFETY DATA SHEET SDS BENZENE 1. Besley and Bottomley 1974.
Closed containers may rupture. Benzene - Density and Specific Weight vs. So the solute molar fraction is 1566 176810-2.
Vapour may travel a considerable distance to a source of ignition and flash back to a leak or open container. Let the vapour pressure pure vapour pressure and mole fraction of component A be P A P A 0 and x A respectively and that of component B be P B P B 0 and x B respectively. At room temperature and pressure the water jar reaches equilibrium when the air over the water has a humidity of about 3.
Indoor VOCs are usually emitted from consumer products and building materials such as paints and carpets and may adversely impact the health of people that are exposed.

The Vapour Pressure Of Pure Benzene And Toluene Are 160 And 60 Mm Youtube
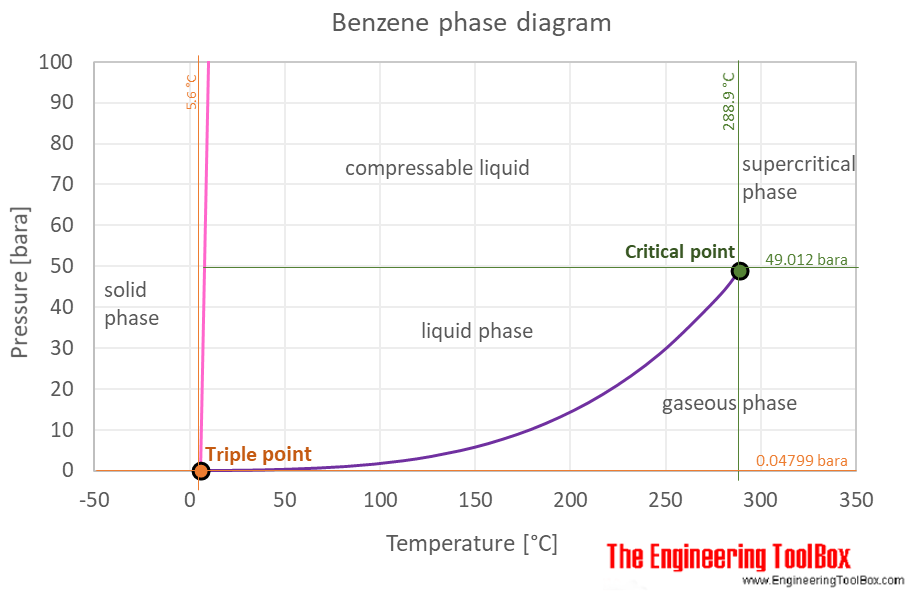
Benzene Thermophysical Properties
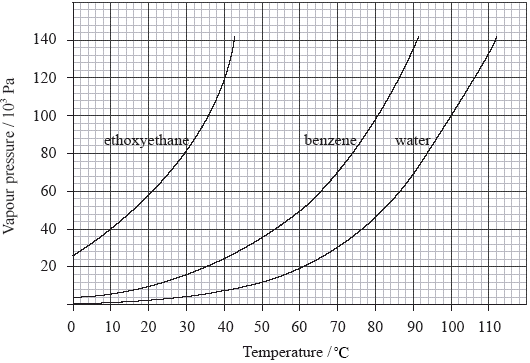
Sl Bonding Practice Exam Question Chemistry Ib Survival

Vapor Pressure Of Benzene Line Experimental Values 34 Circles Download Scientific Diagram
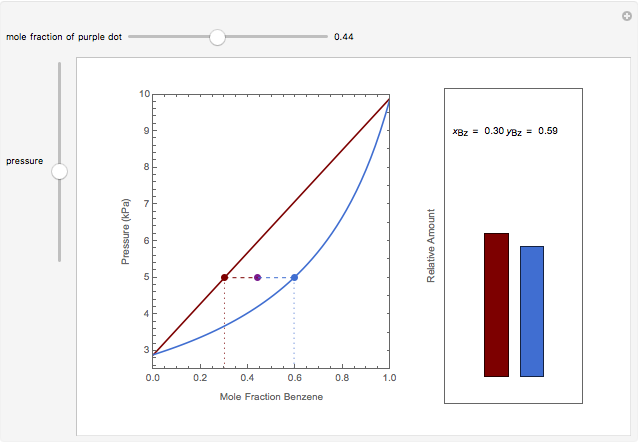
Lever Rule Applied To The Benzene Toluene Vapor Pressure Diagram Wolfram Demonstrations Project

The Vapour Pressure Of Benzene At 80 C Is Lowered By 10mm By Dissoving 2g Of A Youtube

A Vapor Pressure And B Adsorption Amount Of Benzene Dependent On Download Scientific Diagram